| [Molecules: 11] [Related articles/posters: 054 083 112 009 072 ] |
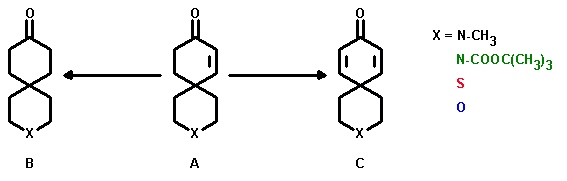
A key reaction step in the synthesis of the 3-heterospiro[5.5]undec-7-en-9-ones (A) is the Robinson annulation of an aliphatic 4-substituted heteroatom-containing aldehyde (D) with methylvinylketone.
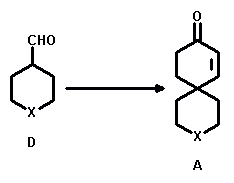
The preparation of these 4-substituted heteroatom-containing aldehydes (D) required as starting materials also turned out to be a synthetic challenge.
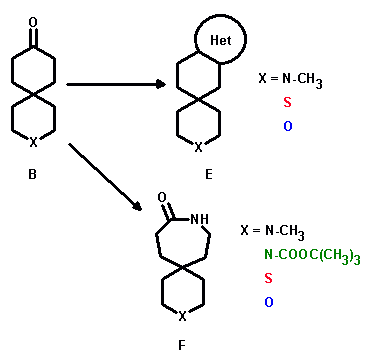
The 3-heterospiro[5.5]undecan-9-ones (B) obtainable from their enone-precursors served as starting materials in the synthesis of various heterocycles (E) as well as substrates for a Schmidt rearrangement to yield 3-hetero-9-azaspiro[5.6]dodecan-10-ones (F).
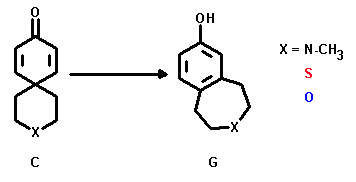
The 3-heterospiro[5.5]undec-7,10-dien-9-ones (C) underwent a dienone-phenol rearrangement to yield benzo[d]heteroepan-7-oles (G) under strong acidic conditions.