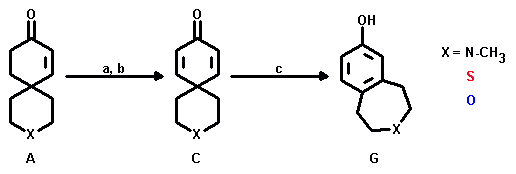
a: PhSeCl, LDA/THF, -80 °C; b: H2O2; yield: 35 - 70% ;
c: X = N-CH3: 9N H2SO4, 10 d; yield: 40%;
X = S: 6N H2SO4, 10 d; yield: 40%;
X = O: 3N H2SO4, 10 d; yield: 40%

a: PhSeCl, LDA/THF, -80 °C; b: H2O2; yield: 35 - 70% ;
c: X = N-CH3: 9N H2SO4, 10 d; yield: 40%;
X = S: 6N H2SO4, 10 d; yield: 40%;
X = O: 3N H2SO4, 10 d; yield: 40%