Preparation of aliphatic 4-substituted heteroatom-containing aldehydes
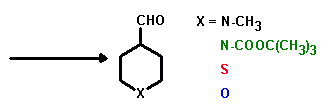
Preparation of 1-methyl-4-piperidinecarboxaldehyde
The preparation of 1-methyl-4-piperidinecarboxaldehyde (3) starting from
1-methylpiperidin-4-one (1) was achieved via a new synthetic pathway. Thus,
1-methylpiperidin-4-one (1) underwent a Wittig-reaction with
(methoxymethyl)triphenylphosphonium chloride in THF solution at -60 °C to yield
4-(methoxymethylene)-1-methylpiperidine (2), which was hydrolysed in acidic solution
to give the aldehyde in an overall high yield (68%).
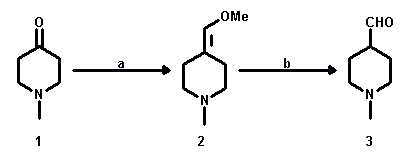
a: Ph3PCH2OCH3Cl, ButOK/THF, -60 °C;
yield: 79%; b: HCl/THF; yield: 87%
This new pathway for the synthesis of 1-methyl-4-piperidinecarboxaldehyde (3) showed
several advantages:
1-Methyl-4-piperidinecarboxaldehyde (3) is not too stable a compound once isolated and
therefore cannot be stored for long periods of time, but
4-(methoxymethylene)-1-methylpiperidine (2) is sufficiently stable not
to decompose upon storage in a freezer for a long time. Furthermore, it was easily
possible to produce 4-(methoxymethylene)-1-methylpiperidine (2) on a reasonable scale
(20-25 g).
Preparation of 4-Formyl-1-piperidinecarboxylic acid 1,1-dimethylethylester
4-Formyl-1-piperidinecarboxylic acid 1,1-dimethylethylester (7) was prepared via
a pathway according to patent literature [1] as outlined below.
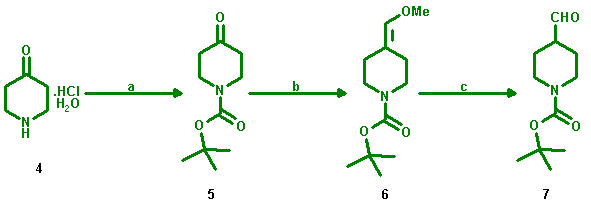
a: Di-tert-butyl dicarbonate, 1 M NaOH/THF; yield: quantitative; b:
Ph3PCH2OCH3Cl, ButOK/THF, -60 °C; yield: 88%;
c: HCl/THF; yield: 77%
Preparation of 2H-Tetrahydrothiopyran-4-carboxaldehyde
For the synthesis of 2H-tetrahydrothiopyran-4-carboxaldehyde (10) two new
synthetic methods were developed. Utilizing 4H-tetrahydrothiopyran-4-one (8)
as starting product 2H-tetrahydrothiopyran-4-carboxaldehyde (10) was approached
by the Lewis acid promoted ring-opening of 1-oxa-6-thiaspiro[2.5]octane (9)
[2].
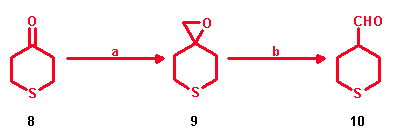
a: (CH3)3SOJ, NaH/DMSO; yield: 77%
b: I. Electrochemical conversion: LiClO4/CH2Cl2, THF, 12 V, 0 °C, Pt-anode, Ni-cathode; yield: 78%;
II. LiJ/THF; yield: 40%
Various commonly used Lewis acids do not yield the desired
product, only after detailed experimental studies could appropriate reaction conditions
be developed.
I. Electrochemical conversion [3]
Stirring the oxirane in a preelectrolysed solution of lithium perchlorate in a
mixture of methylenechloride/THF (5 h electrolysis at 12 V and 0 °C, Pt-anode, Ni-cathode)
at room temp. gave the desired product in excellent yield (78%). A major disadvantage
of this method is the limiting factor of the apparatus only allowing small-scale experiments.
II. Rearrangement with lithium iodide in THF
Lithium iodide, highly concentrated and used in a large excess in THF, turned out to be
the only Lewis type salt to promote the rearrangement of 1-oxa-6-thiaspiro[2.5]octane (9)
to 2H-tetrahydrothiopyran-4-carboxaldehyde (10) in reasonable yield (40%).
Preparation of 2H-Tetrahydropyran-4-carboxaldehyde
A different synthetic approach was set towards the preparation of
2H-tetrahydropyran-4-carboxaldehyde (14) [4] as in
this case no ketone was used as starting material, but 4H-tetrahydropyran-4,4-dicarboxylic
acid diethylester (11), readily available from di(2-chloroethyl) ether and malonic acid
diethylester. By saponification and decarboxylation of 4H-tetrahydropyran-4,4-dicarboxylic
acid diethylester (11) - without isolation of the intermediate
4H-tetrahydropyran-4,4-dicarboxylic acid - 2H-tetrahydropyran-4-carboxylic acid (12) was
prepared, which was then reduced to 2H-tetrahydropyran-4-methanol (13). By subsequent
Swern oxidation 2H-tetrahydropyran-4-carboxaldehyde (14) was made available.
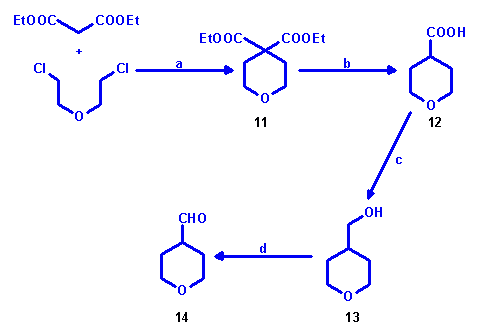
a: NaOEt/EtOH; yield: 62%; b: 6 M HCl; yield: 80%; c: LiAlH4/THF; yield:
70%; d: (COCl)2, DMSO, Et3N/CH2Cl2, -60 °C;
yield: 70%
Reaction steps a-d were performed by analogy with ref. 4a-d with partial modifications.





