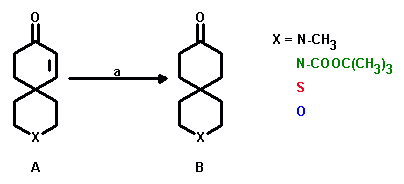
a: H2, Pd/C, EtOAc; yield: 80 - 90%

a: H2, Pd/C, EtOAc; yield: 80 - 90%
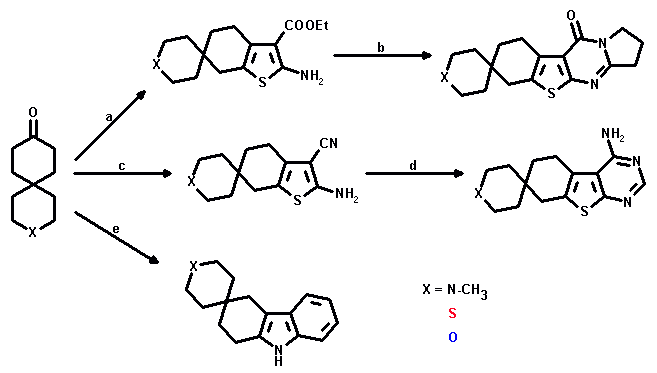
X = N-CH3: a: CN-CH2-COOEt, S, Et2NH/EtOH; yield: 66%;
b: 2-Pyrrolidinone, POCl3/(CH2Cl)2; yield: 57%;
c: CH2(CN)2, S, no base/EtOH; yield: 40%; e: PhNHNH2/AcOH; yield: 38%
X = S: a: CN-CH2-COOEt, S, Et2NH/EtOH; yield: 43%
X = O: a: CN-CH2-COOEt, S, Et2NH/EtOH; yield: 50%;
b: 2-Pyrrolidinone, POCl3/(CH2Cl)2; yield: 39%;
c: CH2(CN)2, S, Et2NH/EtOH; yield: 64%;
d: CHO-NH2, HCOOH/DMF; yield: 67%; e: PhNHNH2/AcOH; yield: 43%
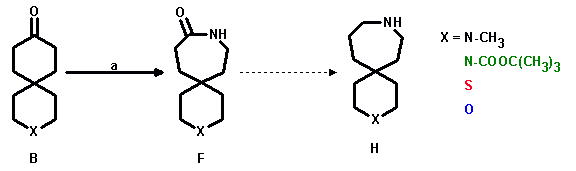
X = S,O: a: H2N-O-SO3H/HCOOH; yield: 30 - 60%
The Schmidt rearrangement worked well for 3-thia- and 3-oxaspiro[5.5]undecan-9-one, whereas for 3-methyl-3-azaspiro[5.5]undecan-9-one (15) the classical Beckmann conditions were applied.
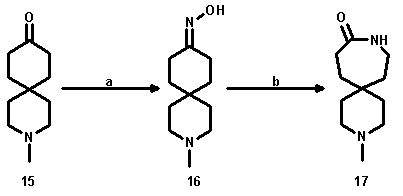
a: H2NOH.HCl, NaOAc/H2O; yield: 67%; b: TosCl/pyridine; yield: 12%
In the case of the Boc-3-azaspiro[5.5]undecan-9-one (18) the protecting group was lost under acidic conditions, but could be attached again.
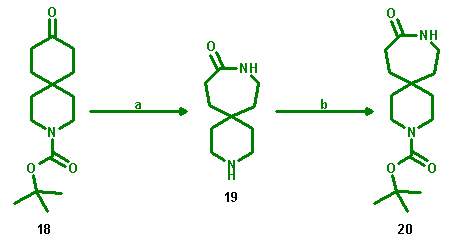
a: H2N-O-SO3H/HCOOH; b: di-tert-butyl dicarbonate, 1 M NaOH/THF