| [Molecules: 10] [Related articles/posters: 083 085 112 054 060 ] |

Dipartimento di Scienze dei Materiali e della Terra Università degli Studi di Ancona Via Brecce Bianche 60131 Ancona, ItalyE-mail
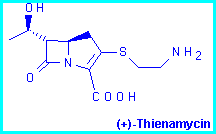
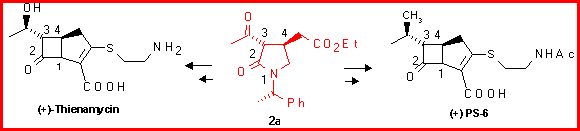
As part of a project [1] aimed at synthesizing enantiomerically pure carbapenem antibiotics, we decided to prepare 3,4-trans-disubstituted pyrrolidin-2-ones which could then be converted by ring contraction into the corresponding 3,4-trans-disubstituted azetidin-2-ones.
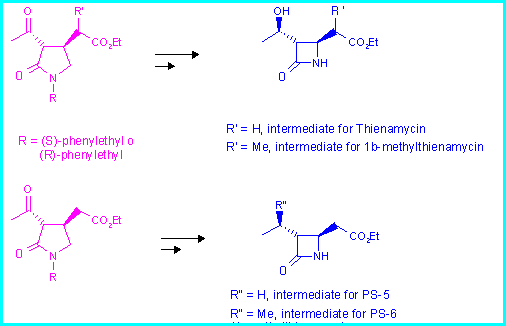
The pyrrolidin-2-ones are obtained by cyclisation of the amides 1a-c by using the conjugate addition reaction.
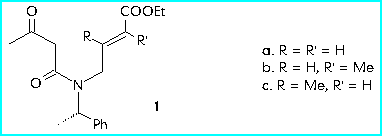
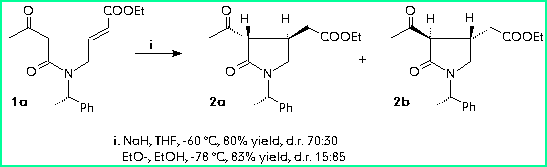
In order to optimize the synthesis of the diastereomer with the correct configuration at the centres 3 and 4, starting from (R)-phenylethylamine, we performed the reaction under conditions giving the best d.r.
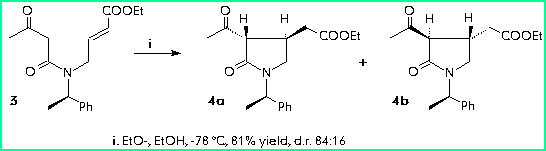
In this way the acetacetamide 3 containing the (R)-phenylethylamine moiety underwent conjugate addition with EtO- in EtOH at - 78 °C, to give an easily separable diastereomeric mixture 4a + 4b in 81% yield and 84:16 d.r.
The reduction of the keto group in 4a was then performed with high diastereoselectivity (95:5) using KBH4 [2], and the stereochemistry of the newly formed stereogenic centre was assigned as (S)- by X-ray analysis of the corresponding p-iodobenzoate ester.
The conjugate addition performed starting from 1b gave the same results observed for compound 1a. In fact the d.r. inverts on changing the reaction conditions from NaH/THF -60 °C to EtO-/EtOH -78 °C (d.r.70:30/15:85).
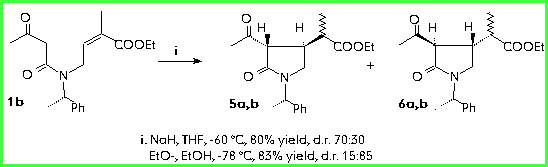
The reaction proceeds with good distereoselection in forming the centres in 3 and 4 (as observed for the cyclisation of compounds 1a), although this does not allow us to control the stereochemistry of the centre in 1". This is not a problem since the conversion of a methyl (in an a:b mixture) into a methylene terminal group has previously been reported [3]. The reaction is performed by using Se derivatives subsequentely transformed into the methylene group by elimination. Catalytic hydrogenation gives the desired 1 b-methyl group.
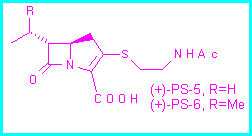
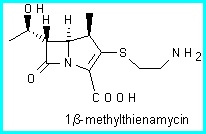
Starting from 5a-b it is possible to synthesize 1 b-methyl carbapenems that are even more active than thienamycin because of its particularly strained structure. In fact the 1 b-methyl group is endo with respect to the bicyclic system.
It is noteworthy that the reduction performed on 5a-b with KBH4 affords a 95:5 diastereomeric mixture as observed for 4a.
Finally, the reaction, performed starting from 1c, afforded an unseparable mixture of four diastereomers as determined from the peaks of the methyl at C-4 in the 1H NMR spectrum, although the configuration of the single components were not assigned.
In conclusion, a synthesis of 3,4-trans-disubstituted pyrrolidin-2-ones was realized with high stereocontrol at either C-3 and C-4, leading to products having the same configuration of the azetidin-2-one ring of carbapenems. In addition a stereoselective reduction of the keto group in the chain at C-3 has been realized. The ring contraction to b-lactams is currently under investigation and will be reported in due course.
References