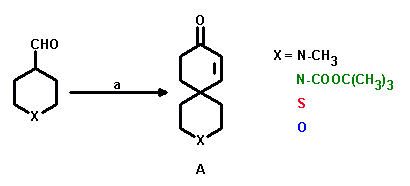
a: X = N-CH3, N-COOC(CH3)3, S: MVK, Triton B/ButOH; yields: 40 - 50%; X = O: MVK, H3PO4/benzene; yield: 70%

a: X = N-CH3, N-COOC(CH3)3, S: MVK, Triton B/ButOH; yields: 40 - 50%; X = O: MVK, H3PO4/benzene; yield: 70%
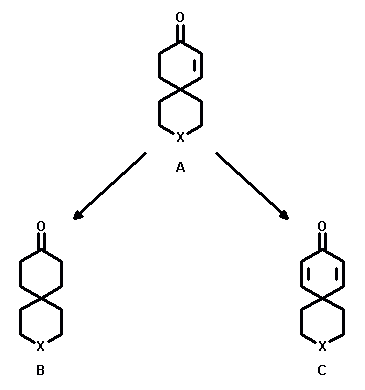
3-Heterospiro[5.5]undec-7-en-9-ones (A) serve as key intermediates. From this class of compounds ketones (B) are accessible via reduction methodology and dienones (C) are accessible via oxidation methodology. Both types of compounds, ketones and dienones, enable transformations to various heterocyclic target systems.