First halogen dance reactions and their selective prevention on bromofurans
Institute of Organic Chemistry, Technical University of Vienna,
Getreidemarkt 9, A-1060 Vienna, Austria
Introduction
It is known that (hetero-)aryl bromides and iodides, which meet certain structural requirements, can undergo a rearrangement
of their substitution pattern upon treatment with alkali amide bases, usually referred to as halogen migration, halogen
dance (HD) or base catalysed halogen dance (BCHD) reaction [1]. Using lithium dialkylamide as base and
quenching the reaction with an electrophile allows the introduction of an additional substituent into the aromatic systems
by means of this reaction type (pathway A). Furthermore, in some cases it is possible to alternatively introduce the
substituent without rearranging the position of the halogen in the same starting compound (prevention of halogen dance,
pathway B).
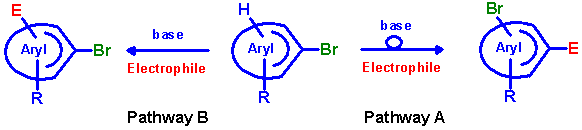
Herein we present the application of both of these reaction types at appropriately substituted bromofurans.
Mechanism
This section shows the mechanism of a halogen dance reaction using a substituted 2-bromofuran as an example. Based on the knowledge about the individual steps involved in the complex sequence of a halogen migration,
the factors essential for selective control of halogen dance or its prevention are explained.
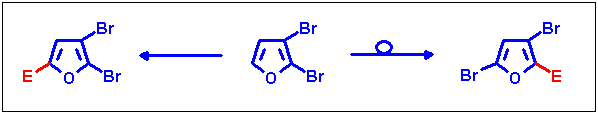
2,3-Dibromofuran as the starting compound
Both halogen migration and prevention of halogen dance could be obtained by variation of the reaction conditions
from this educt, thus providing access to two different substitution patterns via one-pot procedures. These results are
in press[11].
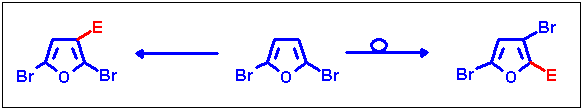
Reactions on 2,5-dibromofuran
Halogen migration was also achieved on the 2,5-isomer, although the substitution pattern was already made accessible
from 2,3-dibromofuran, as this is a different approach for the same system. Prevention of halogen migration here provides
access to another pattern of substituted bromofurans. As with the 2,3-isomer, this work is in press[11].
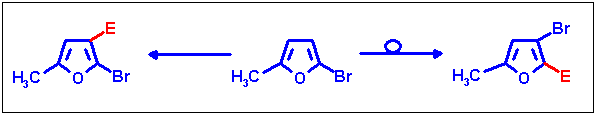
Migration and prevention on 2-bromo-5-methylfuran
2-Bromo-5-methylfuran turned out to be a by far more difficult educt for these reactions, but we managed to provide
selective access to both substitution patterns derived from it. These results have recently been published
[7].
NMR spectroscopy
In addition to the trisubstituted furans prepared via the herein described methods, a large number of mono- and
di-substituted furans was analysed by 1H and 13C NMR spectroscopy. Increments were calculated from
these spectra which were used to prove the structure elucidation of all reaction products on a spectroscopic basis.
Additionally, 17O NMR spectra of all furans were recorded and an increment system for the furan oxygen atom was
developed from these data. An example for the accuracy of the calculation of furan 17O shifts by this system
is given.
Summary
An overview of all halogen dance and migration prevention reactions and the substitution patterns made accessible is given
here.
References
- Substituted Heterocyclic Compounds by Selective Control of Halogen-Dance Reactions,
J. Fröhlich, Progr. Heterocycl. Chem., 1994, 6, 1.
- Synthesis of 2-Substituted 3,5-Dibromothiophenes via a Rearrangement Reaction: A New Example of a Base-Catalyzed Halogen Dance Reaction,
F.Sauter, H.Fröhlich and W.Kalt, Synthesis, 1989, 10, 771.
- Base Catalyzed Halogen Dance Reaction at Thiophenes: A Spectroscopic Reinvestigation of the Synthesis of 2,5-Dibromo-3-trimethylsilyl-thiophene,
H.Fröhlich and W.Kalt, J. Org. Chem. , 1990, 55, 2993.
- Synthesis of Trisubstituted Thiophenes via a Halogen Dance Reaction at 2-Bromo-5-methylthiophene,
J. Fröhlich, C. Hametner and W. Kalt, Monatsh. f. Chemie, 1996, 127, 325.
- B. Majoie, Ger. Offen. 2.030.625 (CI.C07d), 7.Jan. 1971
- Convenient Synthetic Procedures for 2-Bromofuran and 2,5-Dibromofuran, M.A. Keegstra, A.J.A. Klomp and L. Brandsma, Synth. Commun., 1990, 20, 3371.
- Synthesis of Trisubstituted Furans from 2-Bromo-5-methylfuran via Halogen Migrations and Their Selective Preventions, J. Fröhlich and C. Hametner, Monatsh. f. Chemie , 1996, 127, 434.
- Facile isomerization of oxiranes to allyl alcohols by mixed metal bases,
A. Mordini, E. Ben Rayana, C. Margot and M. Schlosser, Tetrahedron, 1990, 46, 2401.
- Superbases for organic synthesis,
M. Schlosser, Pure Appl. Chem., 1988, 60, 1627.
- 17O NMR Spectroscopy in Organic Chemistry,
D.W. Boykin, CRC Press, Boca Raton, 1991.
- Trisubstituted Furans via First Application of Halogen Dance Reactions and their Selective Preventions on 2,3- and 2,5-Dibromofuran,
J. Fröhlich, C. Hametner and W. Kalt, J. Org. Chem., in press.



