2,5-Dibromofuran
2,5-Dibromofuran is accessible via direct bromination of furan in dimethylformamide [6].
The remarkably lower acidity of the furan b-protons as compared to the a-position affects both reaction types, so the conditions had to be changed in order to meet the different requirements.
Halogen migration
The halogen dance reaction on 2,5-dibromofuran can be carried out in the same way as on the 2,3-isomer - addition
of educt to 0.85 equiv. LDA, in this case prolonged reaction time of 90 min, addition of the remaining 0.25 equiv. of
LDA and quenching with an electrophile. However, the low acidity of the proton in position 3, which results in a
significantly slower metalation, allows a simplified procedure: quick addition of dibromofuran to 1 equiv. of LDA
provides sufficient contact between metalated and non-metalated educt for the migration to occur.
Since a series of 2-substituted 3,5-dibromofurans has already been synthesized from 2,3-dibromofuran, only two compounds
have been prepared by this modified procedure.
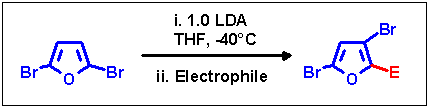
| Electrophile |
E |
Yield (%) |
| Chlorotrimethylsilane |
-Si(CH3)3 |
71 |
| Chlorotrimethylstannane |
-Sn(CH3)3 |
75 |
For the synthesis of trisubstituted derivatives from furan this method is preferred, since 2,5-dibromofuran is accessible
via a simple one-step procedure.
Prevention of halogen dance
The already mentioned lower acidity of the furan b-protons made two changes in the reaction conditions necessary in
order to succeed in selectively retaining halogen migration on 2,5-dibromofuran: the time for the addition of educt to LDA
had to be prolonged to 120 min and the solvent had to be changed to tetrahydropyran (THP), a solvent which we found
to be very efficient and powerful for metalations with LDA [1].
Using this modified method it was possible to provide selective access to 3-substituted 2,5-dibromofurans when using
strong electrophiles. Quenching reagents with weaker electrophilicity gave similar problems as in the prevention reaction
on 2,3-dibromofuran (small amount of rearranged by-product with DMF, unseparable mixtures with alkyl halides).
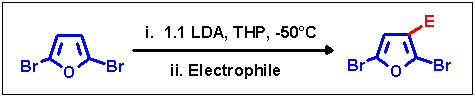
| Electrophile |
E |
Yield (%) |
| Chlorotrimethylsilane |
-Si(CH3)3 |
75 |
| Benzaldehyde |
 |
77 |
| Cyclohexanone |
 |
46 |
| Dimethylformamide |
-CHO |
49 |




