Regioselective photoaddition of amine to styrylthiophenes
T. I. Ho,* C. S. Ho, S. M. Shin and K. Pa
Department of Chemistry,National Taiwan University,Taipei, Taiwan
Abstract
The photochemistry of styrylthiophene(ST) and its derivatives with amines
is investigated. Exciplex emission for tertiary amines and 3-ST systems
have been observed. Photochemical addition of tertiary and secondary amines to
2-ST are non-regioselective. Photoaddition of ammonia to 2-ST sensitized
by dicyano benzene is regioselective. The difference in the photochemical
behaviour is compared.
Styrylthiophene-Amine Exciplexes
The reductive fluorescence quenching of para-substituted trans-3-styrylthioplienes
[X=OCH3, CH3, CH(CH3)2, H, F, Cl, Br, CN] by tertiary amines (triethylamine
TEA, diisopropylmethylamine DIPEA, diisopropylmethylamine DIPMA) were studied.
Exciplexes emission are observed in non-polar solvents. Figure 1a shows
the fluorescence spectra of 3-styrylthiophenes and triethylamine (TEA)
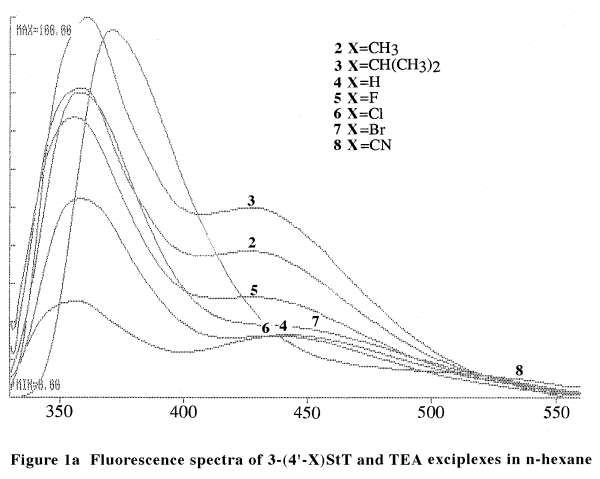
exciplexes in n-hexane. Figure 1b shows the fluorescence spectra of
3-styrylthiophenes and diisopropylmethylamine (DIPMA) exciplexes in benzene.
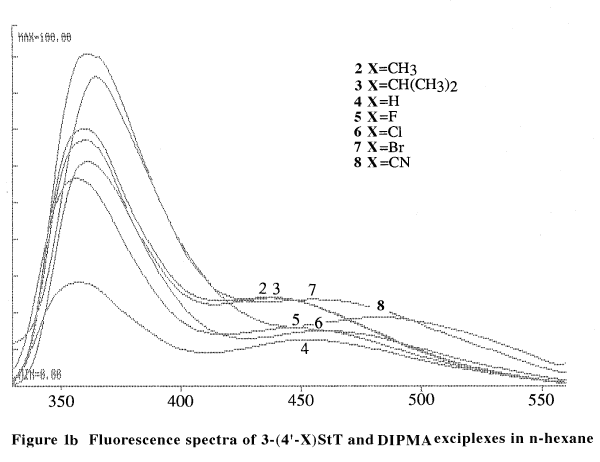
Figure 1c shows the fluorescence spectra of 3-styrylthiophenes and
diisopropylethylamine (DIPEA) exciplexes in benzene. The data are summarized
in Table 1.
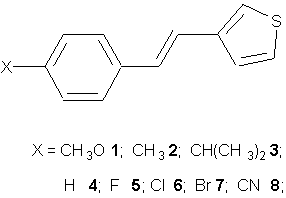
Table 1 Maximum emission of 3-(4'-X-)StT - amine exciplexes
in benzenea and n-hexaneb
| DIPEAa | DIPMAa | TEAb |
| 3-(4'-X-)StT | l(max) | l(max) | lmax |
| X=OCH3 | 414.5 | - | - |
| X=CH3 | 433.0 | 436.2 | 426.2 |
| X=CH(CH3)2 | 434.0 | 437.5 | 427.0 |
| X=H | 442.0 | 450.5 | 438.7 |
| X=F | 453.0 | 446.5 | 427.5 |
| X=Cl | 439.0 | 459.3 | 441.0 |
| X=Br | 499.2 | - | 449.0 |
| X=CN | 2.48 | 436.2 | 480.0 |
Photochemical addition of tertiary amines
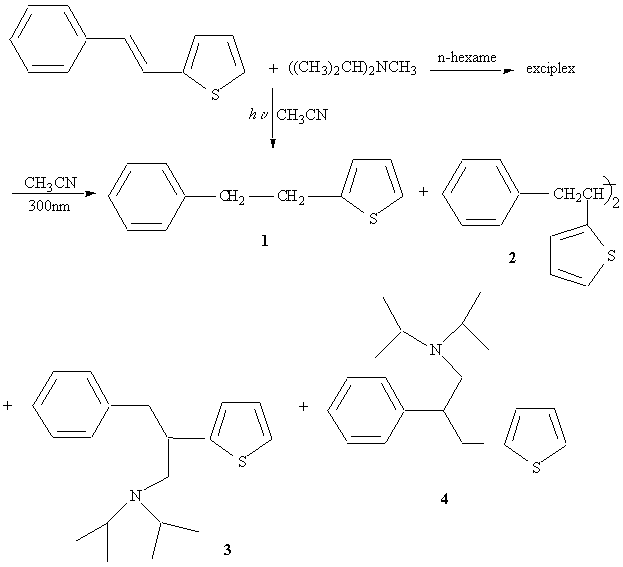
Amine adduct 3 : 4 ratio 1 : 1 nonregioselective
3 + 4 27% yield
Photochemical addition of secondary amines

7 : 8 = 4.5 : 1
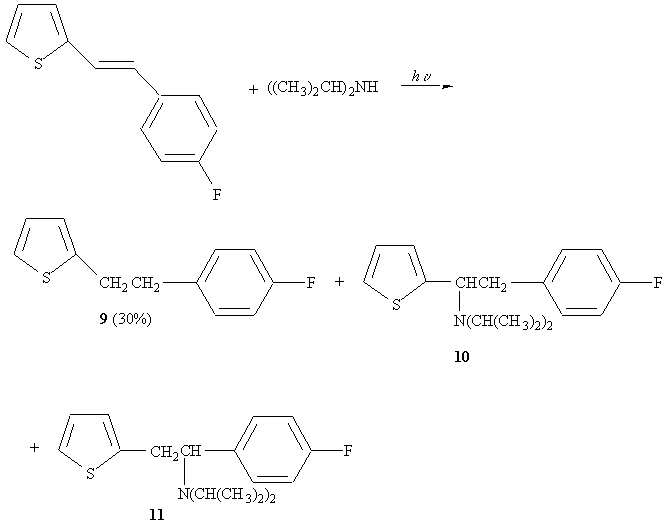
10 : 11 = 3.7 : 1 (~5% yield)
Photochemical addition of ammonia
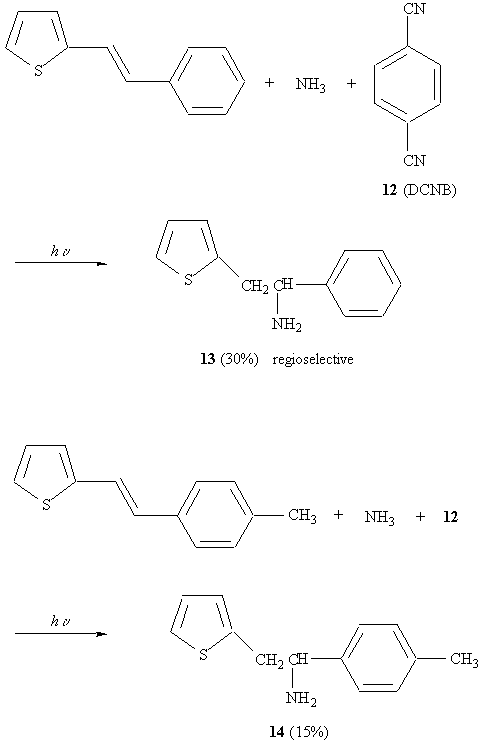
Explanation:
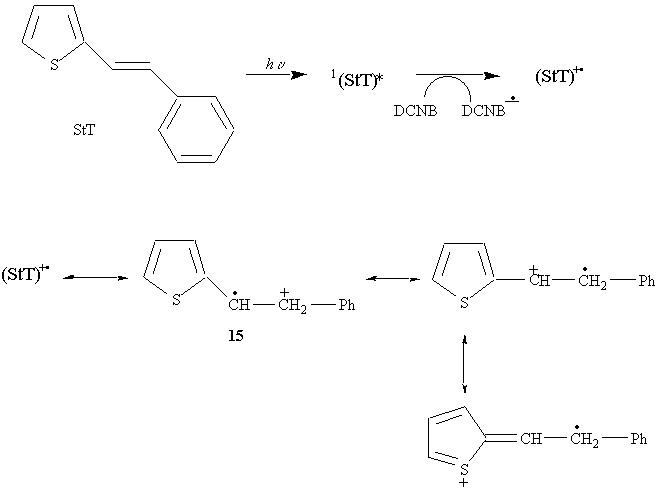
Nucleophilic addition of NH3 only occurs at 15.








