Summary
Halogen migrations and their preventions on dibromofurans and 2-bromo-5-methylfuran can be smoothly controlled
by appropriate reaction conditions: providing sufficient contact between non-metalated and metalated species results
in bromine scrambling with a polybromofuran as key transbromination catalyst, whereas rapidly trapping the starting
material by the metalating agent in a promoting solvent like THP prevents migrations. It has been found that the
reactivity of the electrophile influences the selectivity of HD-prevention reactions due to inducing halogen dance
during the quenching period.
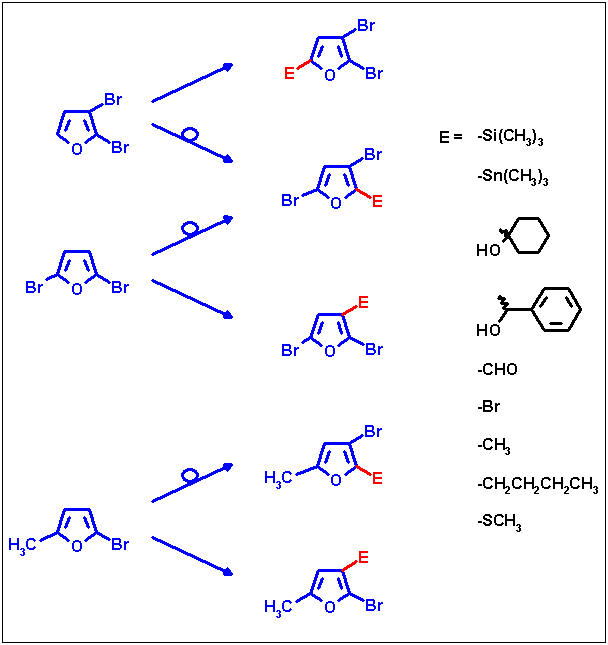
The resulting lithium intermediates can be reacted with various electrophiles thus yielding a number of new
trisubstituted furans. The following scheme shows an overview of substitution patterns made accessible by means of
these two reaction types.
1H and 13C NMR spectroscopy has been used to prove the correctness of all trisubstituted furans
by means of an increment system derived from shifts of mono- and di-substituted compounds. Increments for the furan oxygen
nucleus have been derived from 17O NMR spectra of all furans prepared during this work.

