| [Molecules: 32] [Related articles/posters: 079 025 024 104 066 ] |
Moreover, extension of the synthetic scope by selectively controlling halogen dance vs. its prevention now allows access to a variety of specifically substituted heteroaromatic compounds.
As a result of these studies a series of new tri-substituted thiophenes and furans was obtainable from 2-bromo-(3 or 5)-R-thiophenes or furans (R=Br, CH3, SiMe3, SnBu3). see references
Until now no investigations utilizing "mixed" di-halogenated thiophenes as starting materials for HD chemistry have been made.
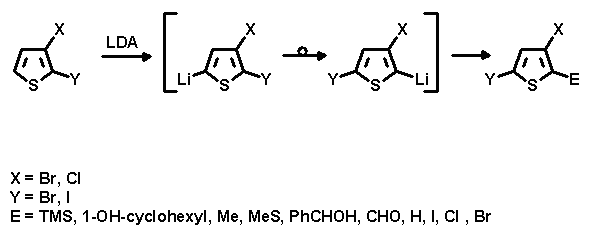
We now report the first sucessful HD reactions of "mixed" halo-substituted thiophenes. As a result we have discovered that the iodo-substituent migrates preferentially over the bromo-substituent, and the bromo-substituent over the chloro-substituent.
The present work aims at selective migration of one of the two halogen atoms followed by subsequent reaction with various electrophiles in order to enable access to new thiophene derivatives with a 2-E-3,5-di-halo substitution pattern.