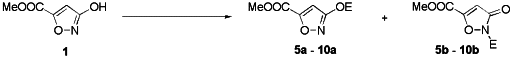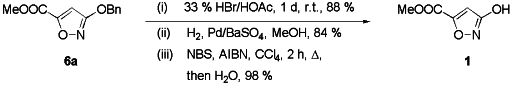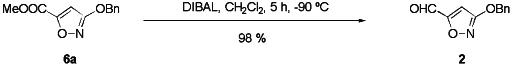New approach
to 3-hydroxyisoxazole-5-carbaldehydes: key intermediates in the synthesis of new
3-hydroxyisoxazoles of pharmacological interest
Michael Schön, Regine Riess, Sabine Laschat and Volker
Jäger*
Institut für Organische Chemie der Universität Stuttgart,
Pfaffenwaldring 55, D-70569 Stuttgart, Germany
Abstract
3-O-Protected 3-hydroxyisoxazole-5-carbaldehydes are
useful building blocks in the synthesis of pharmaceutically interesting
3-hydroxyisoxazoles. We report here our results concerning the synthesis
of such intermediates and their conversion, e.g. into the free vinylene
muscimol, an analogue of g-vinyl-GABA (g-vinyl-g-amino
butyric acid) which represents a strong inhibitor of GABA-transaminase.
Since the discovery of muscimol A1 and ibotenic acid B
- two psychoactive constituents of the mushrooms Amanita muscaria
and A. pantherina - much attention has been focused on the
synthesis of related 3-hydroxyisoxazoles that often display remarkable central nervous system (CNS)
activity2 (see Fig. 1). The most common examples are
4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol C (THIP)1,2f,3
- a bicyclic and less toxic analogue of muscimol - and the glutamic acid
analogue (S)-a-amino-3-(3-hydroxy-4-isoxazolyl)propionic acid
D-[(S)-AMPA].4 THIP C was subjected to clinical
trials with epileptic patients, but no significant effects were
detected.3
The above compounds may be regarded as conformationally restricted bio-isosteres
of neurotransmitters such as g-amino butyric acid (GABA),3
glutamic acid or N-methyl-D-aspartic acid (NMDA).4
Although the 3-hydroxyisoxazole moiety thus represents an important class of
heterocycles, only a few general approaches for their synthesis are known.5
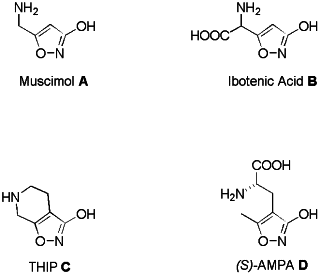 Fig. 1 Examples for naturally occurring or synthetically
3-hydroxy-isoxazoles of pharmaceutical interest
Fig. 1 Examples for naturally occurring or synthetically
3-hydroxy-isoxazoles of pharmaceutical interest
Starting from 3-hydroxyisoxazol 1, as detailed below, we have prepared
the protected 3-hydroxy-isoxazole-5-carbaldehydes 2 and converted them
into the unprotected 3-hydroxyisoxazole-5-carbaldehyde 3 and the
3-hydroxy-isoxazoles vinylene muscimol 4 (see Scheme 1). The aldehyde
3 is presumed to be the major first metabolite of muscimol and is
supposed to cause the pronounced toxicity of this compound.1,6
Vinylene muscimol may be regarded as an analogue of the mechanism-based
inhibitor of GABA transaminase g-vinyl GABA
(Vigabatrin").4,7
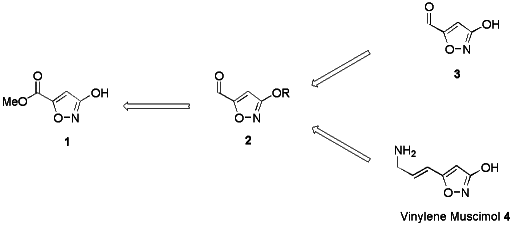
Scheme 1 Retrosynthetic approach to
3-hydroxyisoxazole-5-carbaldehyde 3 and vinylene muscimol 4
3-Hydroxyisoxazole-5-carbaldehydes may be regarded as key intermediates in the
synthesis of these compounds since the aldehyde function allows various
modifications of the side chain. They were used the first time by
Nakamura8 et al. and Krogsgaard-Larsen9 for the synthesis
of ibotenic and homoibotenic acid respectively.
In order to reduce the ester function to the appropriate aldehyde protection of
the hydroxy function of isoxazole 110 is necessary, which is
usually carried out by alkylation of the 3-hydroxyisoxazole-anion. This gives
rise to mixtures of the two regioisomeric O- and N-alkylated
products5,8,9 which have to be separated by chromatography. Another
disadvantage is the harsh conditions required for deprotection (e.g. 33%
HBr/HOAc). The most common OH-protecting group for 3-hydroxyisoxazoles is the
methyl group.2 There are scattered examples for the use of benzyl
(Bn),11 p-methoxybenzyl (PMB),12 methoxymethyl
(MOM),2b,13 phenyl-sulfonyl14 or benzoyl
(Bz)15 groups in this series.
We have examined the suitability of various protecting groups for
3-hydroxyisoxazoles in order to find out whether they could selectively be
introduced at oxygen and removed under mild reaction conditions. In view of
O-selective alkylation of the 3-OH ester 1, we found that only
isoxazoles a could be reduced to the appropriate aldehydes in good
yields. The O-/N-alkylation ratios observed are summarised in
Table 1.
Table 1 O-/N-Alkylation ratios from 3-hydroxyisoxazole
1
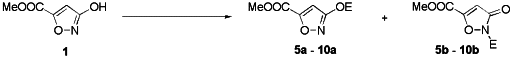
|
E |
Reagents and conditions |
a:ba |
Yields (%)b |
| 1 |
Me |
CH2N2, A |
73:27 |
64 (5a)
25 (5b) |
| 2 |
Bn |
PhCH2Br, B |
94:6 |
93 (6a)
5 (6b) |
| 3 |
Bzh |
Ph2CHBr, B |
> 95:5 |
94 (7a)
0 (7b) |
| 4 |
All |
H2C=CHCH2Br, B |
91:9 |
80 (8a)
8 (8b) |
| 5 |
MOM |
(MeO)2CH2, C |
< 5:95 |
0 (9a)
97 (9b) |
| 6 |
TBDMS |
ButMe2SiCl, D |
> 95:5 |
39 (10a)
0 (10b) |
aRatios determined from the crude products by 13C NMR. bYields
of isolated analytically pure products; Reaction conditions: A Et2O, 0 oC;
B K2CO3, acetone, reflux to room temp.; C P4O10, CHCl3, room temp.; D DBU,
CH2Cl2, room temp.
The highest O-/N-alkylation ratio was obtained in the case of
benzhydryl group16 (entry 3) under typical SN1 conditions. The allyl
and benzyl reagents proved less regioselective. However, in contrast to the
excellent O-/N-alkylation ratio for the introduction of the
benzhydryl group16 (entry 3), the use of the somewhat less effective
benzyl group16 (entry 2) proved preferable since the Bzh-group
showed a tendency to migrate in some of the subsequent reactions applied. In
the case of the TBDMS16 group, high regioselectivity was observed
for the introduction step (entry 6) but accompanied low yield. An example for a
protecting group which could be introduced selectively at nitrogen is the
methoxymethyl moiety16 (entry 5), however the subsequent reduction
to obtain the respective aldehyde failed.
With a viable method to protect the OH-function of isoxazole 1 with high
regioselectivity and in good yield at hand, we explored appropriate methods for
deprotection (see Scheme 2), which was successful for free variations.
- Acidic hydrolysis of the ester 6a using 33 % HBr/HOAc gave the free
hydroxyisoxazole 1 in good yield. These conditions are similar to those
applied for deprotection of 3-methoxyisoxazoles.2
- Catalytic hydrogenation: Although few examples are known in
literature,11 hydrogenative cleavage of the O-Bn bond proved
troublesome at first; however, using Rosenmundt's catalyst (Pd/BaSO4) gave
no acyclic side-products and the free hydroxyisoxazole 1 was isolated in
excellent yield.17
- Alternatively, deprotection of ester 6a with NBS using Anson's
method18 was also successful, see Scheme 2.
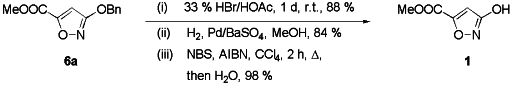
Scheme 2 Deprotection of hydroxyisoxazole 6a
Having established selective O-benzylation and efficient deprotection
conditions for 1/6a, we turned our attention to the preparation
of the free aldehyde 3 (see Scheme 3). Indeed, the pure
O-benzyl-protected hydroxyisoxazole 6a could readily be reduced
to the corresponding aldehyde 2 by DIBAL:19
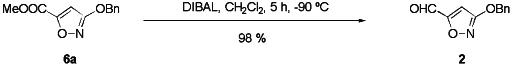
Subsequent deprotection of 2 using 33 % HBr/HOAc was successful likewise
and furnished the target compound 3-hydroxyisoxazole-5-carbaldehyde 3 in
61% yield, see Scheme 3.20
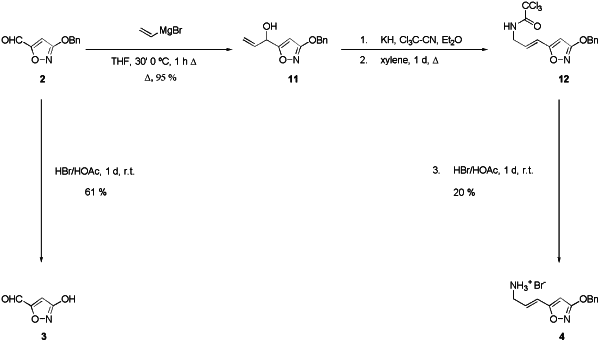
Scheme 3 Synthesis of 3-hydroxyisoxazole-5-carbaldehyde (3)
and vinylene muscimol 4
For the synthesis of vinylene muscimol 4, the aldehyde 2
was transformed into the allylic alcohol 11 by Grignard reaction.
Subsequent Overman rearrangement21 to give the trichloroacetimidate
12 and deprotection under acidic conditions led to the hydrobromide of
vinylene muscimol 4 in poor yield, probably due to the harsh
rearrangement conditions. Hydrogenation of 12 using Pd/BaSO4 as a
catalyst had proved troublesome since partial hydrogenation of carbon-carbon
double bond occurred.
Conclusion
We have demonstrated a new, versatile approach towards known and
new 3-hydroxyisoxazoles of pharmaceutical interest using protected
3-hydroxyisoxazole-5-carbaldehyde 2 as key intermediate. This aldehyde
is now readily available in gram quantities in two steps (91% overall yield)
starting from the known isoxazole 1.10 Further studies
applying this strategy, e.g. for the synthesis of homoibotenic acid derivatives
are in progress.
Acknowledgements
We are grateful to Fonds der Chemischen Industrie for a
fellowship (R. R.) and general support and to Hoechst AG (Dr Gebert, Dr.
Mildenberger) for carrying out some biological tests (no significant activity
detected).
- For reviews on muscimol and related compounds see (a) Krogsgaard-Larsen, P.; Brehm, L.; Schaumburg, K. Acta Chem. Scand.
B 1981, 35, 311. (b) De Feudis, F. V. Rev. Pure Appl.
Pharmacol. Sci. 1982, 3, 319.
- Recent articles included: (a) Madsen, U.; Frydenvang, K.; Ebert, B.;
Johansen, T. N.; Brehm, L.; Krogsgaard-Larsen, P. J. Med. Chem.
1996, 39,183. (b) Skjoerboek, N.; Ebert, B.; Falch, E.; Brehm,
L.; Krogsgaard-Larsen, P. J. Chem. Soc. Perkin Trans. I, 1995,
221. (c) Ebert, B.; Lenz, S.; Brehm, L.; Bregnedal, P.; Hansen, J. J.;
Frederiksen, K.; Bøgesø, K. P.; Krogsgaard-Larsen, P. J. Med.
Chem. 1994, 37, 878. (d) Johansen, T. N.; Frydenvang,
K.; Ebert, B.; Krogsgaard-Larsen, P.; Madsen, U. J. Med. Chem.
1994, 37, 3252. (e) Madsen, U.; Wong, E. H. F. J. Med. Chem.
1992, 35, 107. (f) Hjeds, H.; Christensen, I. T.; Cornett,
C.; Frølund, B.; Falch, E.; Pedersen, J. B.; Krogsgaard-Larsen, P
Acta Chem. Scand. 1992, 46, 772. (g) Brehm, L.; Johansen,
J. S.; Krogsgaard-Larsen, P. J. Chem. Soc. Perkin Trans. I 1992,
2059. (h) Krogsgaard-Larsen, P.; Ferkany, J. W.; Nielsen, E. Ø.; Madsen,
U.; Ebert, B.; Johansen. J. S.; Diemer, N. H.; Bruhn, T.; Beattie, D. T.;
Curtis, D. R. J. Med. Chem. 1991, 34, 123.
- Krogsgaard-Larsen, P.; Frølund, B.; Jørgensen, F. S.;
Schousboe, A. J. Med. Chem. 1994, 37, 2489.
- (a) Krogsgaard-Larsen, P. Comprehensive Med. Chem. 1990,
3, 493. (b) Johnson, R. L.; Koerner, J. F. J. Med. Chem.
1988, 31, 2057.
- Christensen, S. B.; Krogsgaard-Larsen, P. Acta Chem. Scand. B
1974, 38, 625. For synthesis of 3-hydroxyisoxazoles in general see e.g. Lang Jr., S.A.; Lin,
Y.-I. in "Comprehensive Heterocyclic Chemistry", Vol. 6; Katritzky,
A.R.; Rees, C.W., Ed.; Pergamon; London, New York 1984.
- Fowler, L. J.; Lowell, D. H.; John, R. A. J. Neurochem.
1983, 41, 1751.
- (a) Metcalf, B. W.; Jung, M. J.; Lippert, B.; Casara, P.; Böhlen, P.;
Schechter, P. J. in GABA Neurotransmitters; Krogsgaard-Larsen, P.;
Scheel-Krüger, J.; Kofod, H., Ed.; Munksgaard: Copenhagen, 1990.
(b) Allan, R. D.; Johnston, G. A. R. Med. Res. Rev. 1983,
3, 91.
- Kishida, Y.; Hiraoka, T.; Ide, J.; Terada, A.; Nakamura, N. Chem.
Pharm. Bull. 1966, 14, 92.
(9) (a) Hansen, J. J.; Krogsgaard-Larsen, P. J. Chem. Soc. Perkin Trans.
I 1980, 1826. (b) Hansen, J. J.; Krogsgaard-Larsen, P. J. Chem. Soc. Chem. Comm. 1979, 87. (c) Krogsgaard-Larsen, P.; Christensen,
S. B. Acta Chem. Scand. B 1976, 30, 281.
- (a) Bennouna, C.; Petrus, F.; Verducci, J. Bull. Soc. Chim. Fr.
1980, 478. (b) Jäger, V.; Frey, M. Liebigs Ann. Chem.
1982, 817. (c) Frey, M.; Jäger, V. Synthesis 1985,
478.
- Madsen, U.; Brehm, L.; Krogsgaard-Larsen, P. J. Chem. Soc. Perkin
Trans. I 1988, 359.
- Frølund, B.; Kristiansen, U.; Brehm, L.; Hansen, A. B.;
Krogsgaard-Larsen, P.; Falch, E. J. Med. Chem. 1995, 38,
3287.
- Begtrup, M.; Sløk, F. A. Synthesis 1993, 861.
- Nakamura, N.; Tajima, Y.; Sakai, S. Heterocycles 1982,
17, 235.
- Schlewer, G.; Krogsgaard-Larsen, P. Acta Chem. Scand. B
1984, 38, 815.
- Loewenthal, H. J. Q. in Protective Groups in Organic Chemistry;
McOmie, J. F. W. Ed.; Plenum Press: New York, 1981.
- It is noteworthy that the identical reaction conditions applied to the
N-benzyl product 6b furnished the open chain N-benzyl
amide: Schön, M. Dissertation, Universität Stuttgart
(planned).
- Anson, M. S.; Montana, J. G. Synlett 1994, 219.
- Winterfeldt, E. Synthesis 1975, 617.
- Deprotection using H2, Pd/BaSO4 furnished the appropriate benzyl alcohol
in 98% yield: Schön, M. Dissertation, Universität Stuttgart
(planned).
- Overman, L. E. J. Am. Chem. Soc. 1976, 98, 2901.