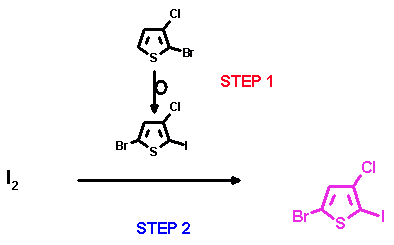

In a first step we rearranged the starting compound under formation of the 2-Li-5-bromo-3-chloro-intermediate, which in a next step was slowly added to iodine in THF, thus immediately quenching the Li-intermediate. As in this case no significant contact between formed target compound and Li-intermediate is made, the desired 5-bromo-3-chloro-2-iodothiophene was obtained exclusively with good yield (70%).