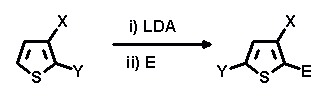
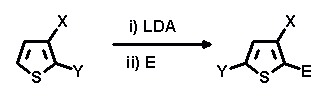
By selective migration of one halogen atom on 2,3-dihalothiophene upon quenching with various electrophiles a series of 2-E-3,5-dihalo-products was obtainable in good yields (54-89%). The scope of the reaction is slightly limited by introducing substituents that can undergo further transhalogenation reactions (i.e. E = Br, I).