

 1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26


![]() (likewise Furan
(likewise Furan![]() and Thiophene)
and Thiophene)
•
its electron lone pair is involved in π cloud
![]() Reactivity and Orientation
Reactivity and Orientation
![]() reaction occur mainly in 2-position
reaction occur mainly in 2-position
extra canonical involved in substitution at the 2-position
•
•
•
•
•
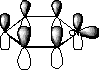
resonance energy 23 kcal/mol
uses only 1![]() electron in π cloud
electron in π cloud
acts as a base
undergoes nucleophilic substitution
highly deactivated (ring nitrogen similar to nitro-substituent); 3-position
![]() Reactivity and Orientation
Reactivity and Orientation
destabilising
![]() no such destabilisation for meta attack!
no such destabilisation for meta attack!
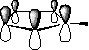
![]() +Cl
+Cl![]() -
-
![]() 2
2
![]() 3
3![]() H
H
![]() 4
4
![]() E+
E+
![]() E+
E+
![]() / H2SO4
/ H2SO4
![]() 300oC
300oC
550![]() oC
oC
![]() Br2
Br2
300![]() oC
oC
![]() AlCl3
AlCl3