

 1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26


reaction conditions used:
•
it reacts faster in α-position (only two possible sites of attack)
![]() these are major contributing canonicals
these are major contributing canonicals
![]() 1
1![]() resonance form which does not destroy the second aromatic
resonance form which does not destroy the second aromatic
ring is available:
![]() interaction with the hydrogen atom at C8
interaction with the hydrogen atom at C8
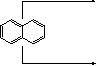

![]() 3H
3H
![]() 3H
3H
![]() 80
80![]() oC
oC
conc. H![]() 2SO
2SO![]() 4
4
![]() 160o
160o![]() C
C
conc. H2![]() SO
SO![]() 4
4
![]() 160o
160o![]() C
C
conc. H2![]() SO4
SO4

![]() H
H
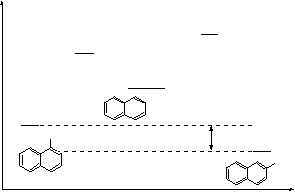
![]() 3H
3H
![]() 3H
3H
![]() SO4
SO4