

 1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26


![]() Friedel-Crafts Acylation
Friedel-Crafts Acylation
•
•
![]() Clemmensen (Zn/HCl)
Clemmensen (Zn/HCl)
BASE:![]() Wolff-Kishner NH2NH2/base
Wolff-Kishner NH2NH2/base
NEUTRAL: Mozingo via ![]() cyclic
cyclic ![]() thioketal
thioketal ![]() followed
followed
Nickel
![]() +
+![]() HX
HX
![]() R'-COX
R'-COX
![]() AlCl
AlCl![]() 4
4
![]() H
H![]() +
+
![]() 3
3
![]() Zn/HCl
Zn/HCl
![]() Zn/HCl
Zn/HCl
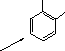
![]() 3
3
![]() 2
2
![]() 3H
3H
![]() CN
CN