

 1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26


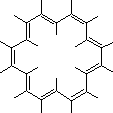
lead to distortion from planarity. Bond lengths 1.35-1.41Å: essentially aromatic.
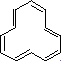
alternating bond lengths, is non-planar and
therefore non-aromatic.
•
generates σ-complex
![]() +
+