1,3-Anionic Cycloreversions of N-lithioimidazolidines:
a new route to 2-azaallyl anions
William H. Pearson,* Michael A. Walters
and William G. Harter
Department of Chemistry, University of Michigan, Ann Arbor, Michigan, USA 48109-1055
We have been studying the generation and cycloaddition of 2-azaallyl
anions [1], a convenient method for the synthesis
of pyrrolidines [2-5]. We have recently been
able to apply this chemistry to the synthesis of various alkaloids [6-8]. We found that the most general method for 2-azaallyl anion generation
is tin-lithium exchange. However, our early work focused on the deprotonation
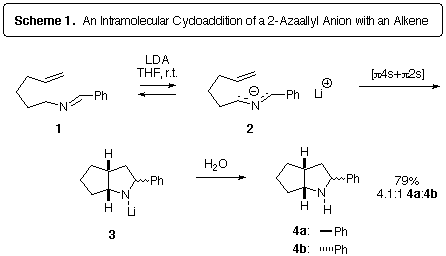
of imines, the method originally reported by Kauffmann [9]. An early example of our work is shown in Scheme 1, which illustrates
the first example of an intramolecular 2-azaallyl anion cycloaddition [2]. Deprotonation of 1 with lithium diisopropylamine (LDA) generated
the 2-azaallyl anion 2, which underwent an anionic cycloaddition
to produce the N-lithiopyrrolidine 3. Quenching the reaction
with water afforded the bicyclic pyrrolidine 4 in good yield.
Ultimately, we found that the deprotonation route to 2-azaallyl anions
was limited to non-enolizable imines, thus precipitating our more recent
excursion into tin-lithium exchange methodology. However, our studies on
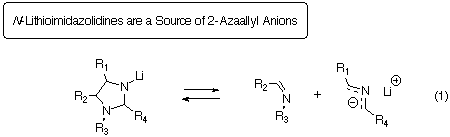
deprotonation led us to discover a new route to 2-azaallyl anions, namely
the 1,3-anionic cycloreversion of N-lithioimidazolidines (eqn. 1).
This paper describes this discovery and the further use of this method.
Perhaps most interesting is that the imidazolidine and deprotonation methods
differ in the stereochemical outcome of the cycloadditions.
- Introduction (This page)
- Observations of imidazolidine intermediates in the
deprotonation route to 2-azaallyl anions
- Deliberate generation of imidazolidines from
2-azaallyl anions, and their use as 2-azaallyl anion precursors
- Synthesis of imidazolidines from diamines, and
their use in 2-azaallyl anion generation
- An unusual stereochemical complementarity
- How general is the imidazolidine fragmentation
route to 2-azaallyl anions? and Conclusion
- Experimental
- References

