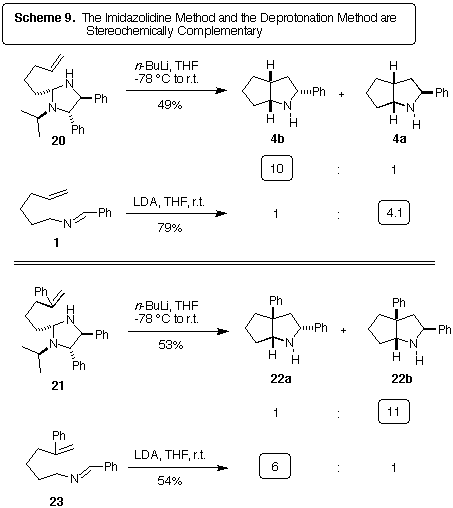
An unusual stereochemical complementarity
Scheme 9 summarizes the stereochemical outcome of the imidazolidine method
and the deprotonation method for 2-azaallyl anion synthesis. Note that
in each case, the two routes give the opposite stereochemical outcomes.
Pyrrolidines 4b and 22a are the likely result of cycloaddition
through the 'W'-geometry of the 2-azaallyl anion, while pyrrolidines
4a and 22b must proceed through a 'sickle'-geometry.
It is certainly believable that the imidazolidine method and the deprotonation
method may give different anion geometries. However, there are several
curious aspects to this hypothesis. First, as determined above, the deprotonation
of 1 goes through imidazolidines! Why, then, would these imidazolidines
and 20 give different results? Possible explanations include
- Imidazolidines 7 and 8 (Scheme 3) each
produce different 2-azaallyl anion geometries
- The stereochemistry
of 20 [particularly at C(2)] is different from the stereochemistry
of imidazolidine 7 (Scheme 3), perhaps leading
to a different 2-azaallyl anion geometry.
Where the explanation becomes
difficult is with the results shown at the bottom of Scheme 9. Note that 21,
an imidazolidine analogous to 20, gives products resulting from the
sickle- rather than W-anion geometry. How can the simple change of placing
a phenyl group on the anionophile cause such a reversal? Also surprising
is the fact that the deprotonation of 23 gives opposite results to
the deprotonation of 1. However, no evidence for imidazolidine intermediates
was obtained in the deprotonation of 23, so these two experiments
are not entirely comparable.
The stereochemistry of the bicyclic pyrrolidines was vouchsafed by difference
NOE NMR experiments.
1. Introduction
2. Observations of Imidazolidine Intermediates in the
Deprotonation Route to 2-Azaallyl Anions
3. Deliberate Generation of Imidazolidines from 2-Azaallyl
Anions, and Their Use as 2-Azaallyl Anion Precursors
4. Synthesis of Imidazolidines from Diamines, and
Their Use in 2-Azaallyl Anion Generation
5. An Unusual Stereochemical Complementarity (This page)
6. How General is the Imidazolidine Fragmentation
Route to 2-Azaallyl Anions? and Conclusion
7. Experimental Section
8. References

