Synthesis of imidazolidines from diamines, and their use in
2-azaallyl anion generation
Since imidazolidines may be prepared from diamines and aldehydes, we sought
to explore this method as a route to 2-azaallyl anions. Initially, we prepared
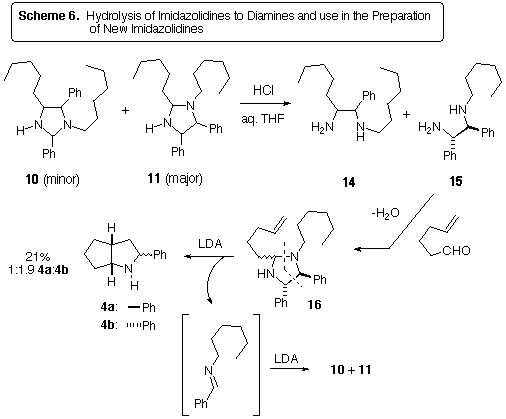
the diamines 14 and 15 from the imidazolidines 10 and
11, since we had them in hand (Scheme 6). The imidazolidines used
in this case were derived from the benzene experiment in Scheme 4. Thus
the diamine 15 was formed as the major product upon acidic hydrolysis.
Condensation of 15 with hex-5-enal in a Dean-Stark apparatus afforded
the imidazolidine 16, which was treated with LDA. Cycloreversion
to the 2-azaallyl anion and N-benzylidinehexylamine occurred, leading
to the bicyclic pyrrolidine 4. Unfortunately, the N-benzylidinehexylamine
by-product dimerized to the imidazolidines 10 and 11, making
the workup and isolation of 4 more difficult. Nonetheless, this
experiment showed that diamines may be used as starting materials for 2-azaallyl
anion chemistry.
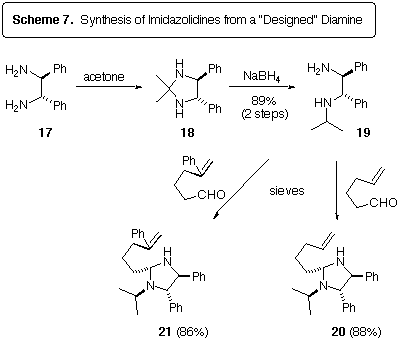
To avoid the dimerization of the imine by-product of the imidazolidine
cycloreversions, we synthesized the N-isopropyl diamine 19
from (d,l)-stilbenediamine 17 [15] by
a reductive amination approach (Scheme 7). We hoped that imidazolidines
derived from 19 would form N-benzylidineisopropylamine as
a by-product, which might not dimerize due to steric considerations. Scheme
7 also shows the successful conversion of 19 to the imidazolidines
20 and 21. The stereochemistry at C(2) of these compounds
is assumed based on molecular modelling (MM2), assuming that the most stable
diastereomer would be formed under the condensation conditions.
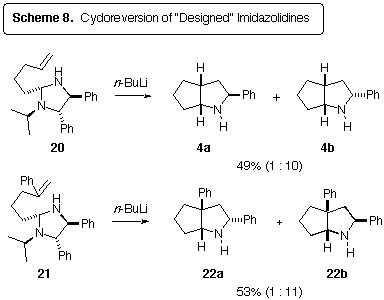
With the imidazolidines 20 and 21 in hand, we subjected
these compounds to deprotonation with butyllithium (Scheme 8).
Smooth cycloreversion of the N-lithioimidazolidines occurred, leading
to the cycloadducts 4 and 22. No messy by-products were observed
from the dimerization of N-benzylidineisopropylamine, which was observed
in nearly quantitative amounts by GC.
- Introduction
-
Observations of imidazolidine intermediates in the
deprotonation route to 2-azaallyl anions
-
Deliberate generation of imidazolidines from 2-azaallyl
anions, and their use as 2-azaallyl anion precursors
-
Synthesis of imidazolidines from diamines, and their use in 2-azaallyl
anion generation (This page)
-
An unusual stereochemical complementarity
-
How general is the imidazolidine fragmentation
route to 2-azaallyl anions? and Conclusion
-
Experimental section
-
References



