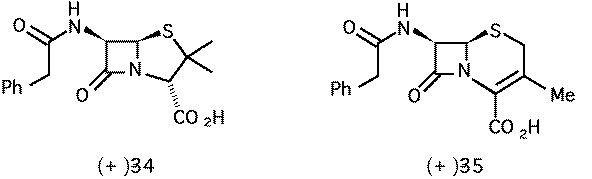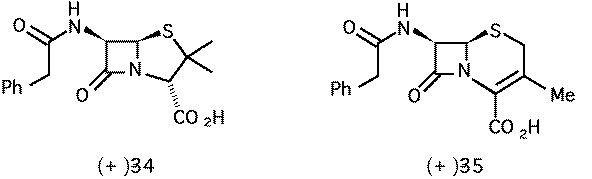New b-lactam analogues as antimicrobial agents: design and synthesis
Reuben Jih-Ru Hwu,* Shwu-Chen Tsay, Zahra Ranezani and Gholam H. Hakimelahi
Organosilicon and Synthesis Laboratory, Institute of Chemistry, Academia Sinica, Nankang, Taipei, Taiwan 11529, ROC and
Department of Chemistry, National Tsing Hua University, Hsinchu, Taiwan 30043, ROC
Abstract
The cis-configurated race-(6RS,7RS)-3-hydroxy-8-oxo-7-(2-phen-
oxyacetamido)-4-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic
acid as well as rac-(6RS,7RS)-3-hydroxy-8-oxo-7-(2-phenoxy-
acetamido)-4-oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic
acid and rac-3-methyl 2-hydrogen (6RS,7RS)-8-oxo-7-(2-
phenylacetamido)-4-oxa-1-azabicyclo[4.2.0]oct-2-ene-2,3-
dicarboxylate were synthesized. The key step involves
chlorination of the corresponding carbonions of rac-tert-
butyl 3-{[cis-4-oxo-3-(2-phenoxyacetamido)azetidin-2-yl]
methylthio}-3-oxopropanoate, rac-tert-butyl [cis-4-oxo-3-
(2-phenoxyacetamido)azetidin-2-yl]methyl propanedioate, and
rac-1-benzyl 4-methyl 2-[cis-4-(hydroxymethyl)-2-oxo-3-
(phenylacetamido)azetidin-1-yl]-3-(phenylsulfonyl)butane-
dioate with CF3SO2Cl followed by internal alkylation. Our
newly synthesized b-lactams were found to possess
biological activity aganist several pathogenic microorganisms
in vivo. The pathogenic microorganisms include S. aureus
(FDA 209P), E. coli (ATCC39188), S. typhi (O-901),
Ps. aeruginosa (1101-75), and K. pneumoniae (NCTC418).Electronic activation of the lactam moiety in rac-3-
methyl 2-hydrogen (6RS,7RS)-8-oxo-7-(2-phenylacetamido)-4-
oxa-1-azabicyclo[4.2.0]oct-2-ene-2,3-dicarboxylate resulted in a remarkably enhanced biological activity.
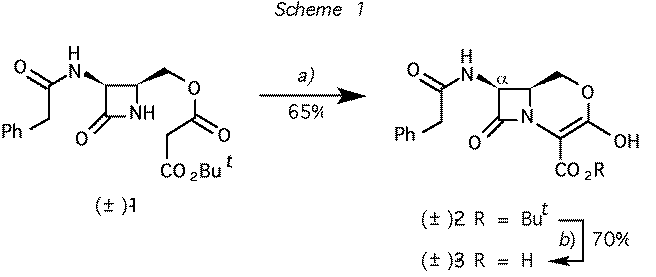
Scheme 1 Reagents and conditions: (a) CF3SO2Cl, Et3N, CH2Cl2. b) CF3CO2H, CH2Cl2, 25 oC
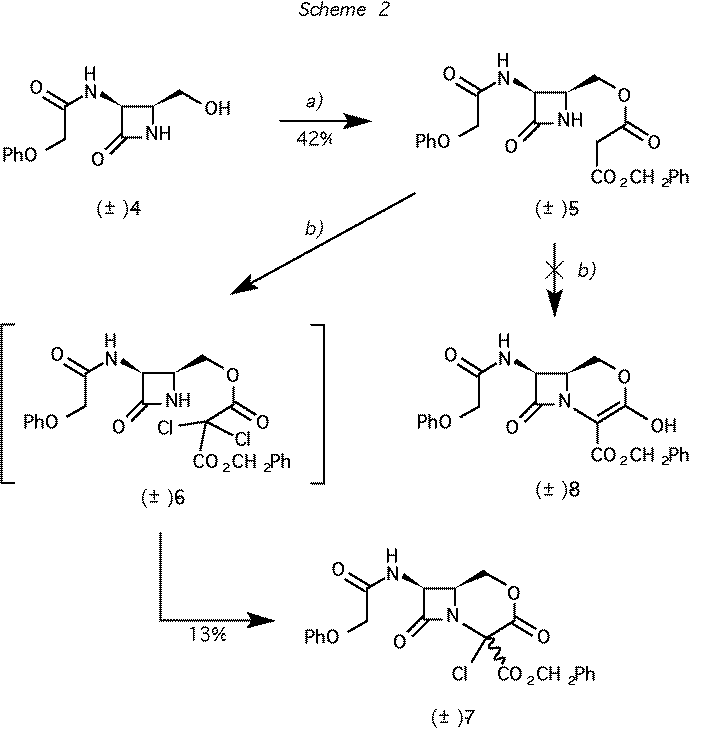
Scheme 2 Reagents and conditions: (a) ClCOCH2CO2CH2Ph, pyridine, CH2Cl2. b) CF3SO2Cl, Et3N,
CH2Cl2.
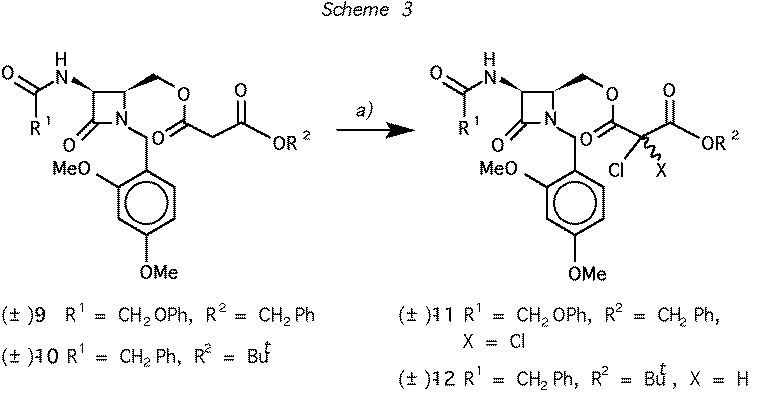 Scheme 3 Reagents and conditions: (a)
CF3SO2Cl, Et3N, CH2Cl2: 90% for 9 -> 11, 80% for 10
-> 12.
Scheme 3 Reagents and conditions: (a)
CF3SO2Cl, Et3N, CH2Cl2: 90% for 9 -> 11, 80% for 10
-> 12.
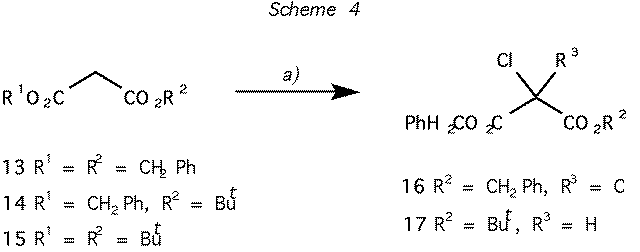
Scheme 4 Reagents and conditions: (a) CF3SO2Cl, Et3N, CH2Cl2: 95% for 13 -> 16, 75% for
14 -> 17, no reaction with 15.
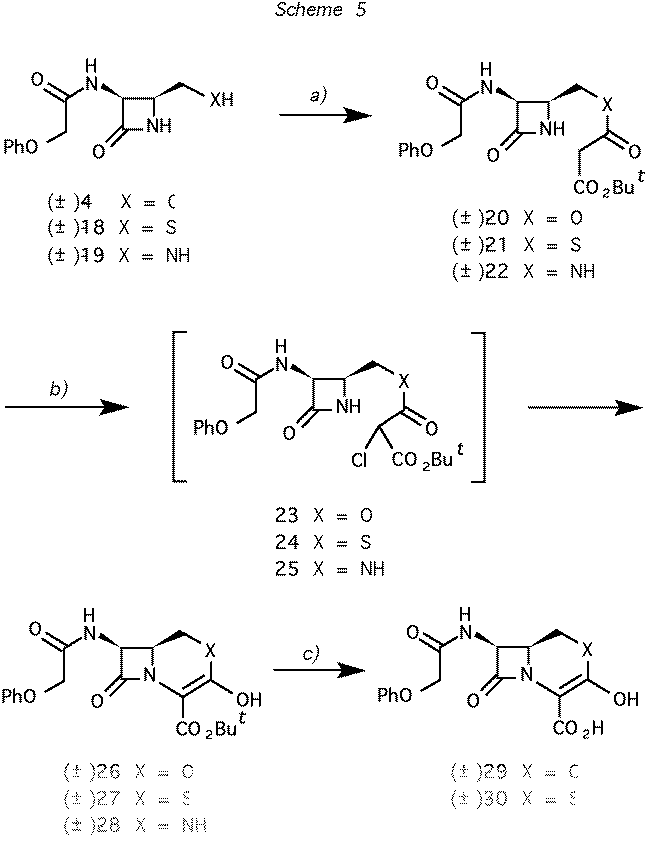
Scheme 5 Reagents and conditions: (a) ClCOCH2CO2But, pyridine, CH2Cl2: 40% for 4
-> 20, 58% for 18 -> 21, 70% for 19 ->
22. b) CF3SO2Cl, Et3N, CH2Cl2: 60% for 20 -> 23
-> 26, 70% for 21 -> 24 -> 27, 46% for
22 -> 25 -> 28. c) Bu4NClO4,
CF3CO2H, CH2Cl2: 80% for 26 -> 29, 85% for 27 ->
30.
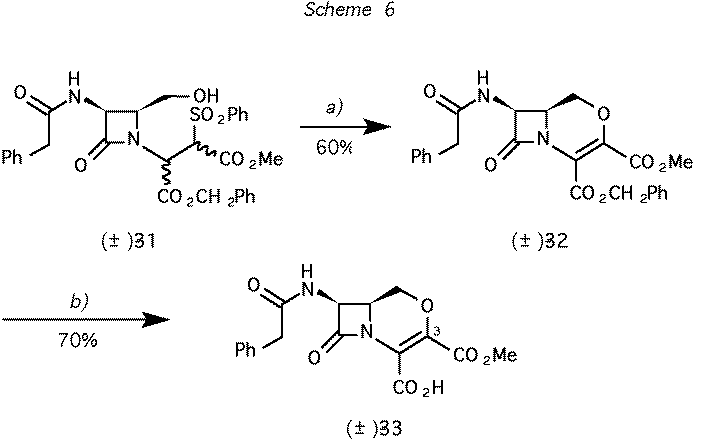
Scheme 6 Reagents and conditions: (a) 1. CF3SO2Cl, Et3N, CH2Cl2; 2. DBU. b) H2 (50 psi), PdCl2,
AcOEt.