Peter Murray-Rust in his blog asks for examples of the Scientific Semantic Web, a topic we have both been banging on about for ten years or more (DOI: 10.1021/ci000406v). What we are seeking of course is an example of how scientific connections have been made using inference logic from semantically rich statements to be found on the Web (ideally connections that might not have previously been spotted by humans, and lie overlooked and unloved in the scientific literature). Its a tough cookie, and I look forward to the examples that Peter identifies. Meanwhile, I thought I might share here a semantically rich molecule. OK, I identified this as such not by using the Web, but as someone who is in the process of delivering an undergraduate lecture course on the topic of conformational analysis. This course takes the form of presenting a set of rules or principles which relate to the conformations of molecules, and which themselves derive from quantum mechanics, and then illustrating them with selected annotated examples. To do this, a great many semantic connections have to be made, and in the current state of play, only a human can really hope to make most of these. We really look to the semantic web as it currently is to perhaps spot a few connections that might have been overlooked in this process. So, below is a molecule, and I have made a few semantic connections for it (but have not actually fully formalised them in this blog; that is a different topic I might return to at some time). I feel in my bones that more connections could be made, and offer the molecule here as the fuse!
WebCite and Jmol
April 18th, 2010Since I have gotten into the habit of quoting some of my posts in other contexts, I have started to also archive them using WebCite. One can quote the resulting archive as:
Carbobenzene: benzene with a difference
April 16th, 2010Some molecules, when you first see them, just intrigue. So it was with carbobenzene, the synthesis of a derivative of which was recently achieved by Remi Chauvin and co-workers (DOI: 10.1002/chem.200601193). Two additional carbon atoms have been inserted into each of the six C-C bonds in benzene.
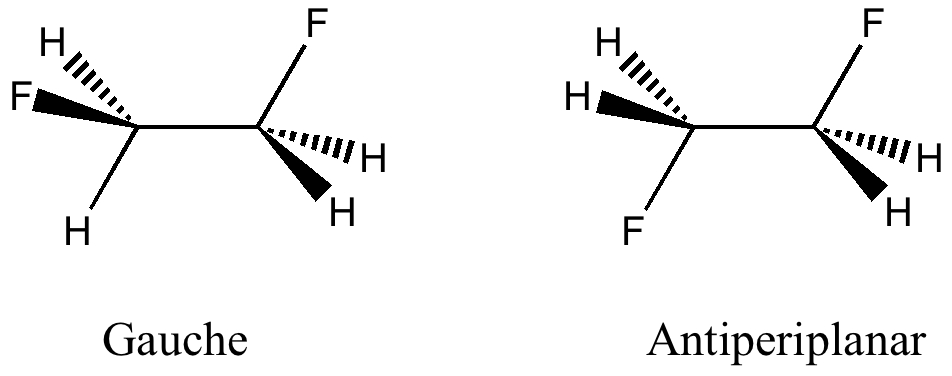
The conformation of 1,2-difluoroethane
April 6th, 2010Here I offer another spin-off from writing a lecture course on conformational analysis. This is the famous example of why 1,2-difluoroethane adopts a gauche rather than antiperiplanar conformation.
The structure of the hydrogen ion in water.
February 21st, 2010Stoyanov, Stoyanova and Reed recently published on the structure of the hydrogen ion in water. Their model was H(H2O)n+, where n=6 (DOI: 10.1021/ja9101826). This suggestion was picked up by Steve Bachrach on his blog, where he added a further three structures to the proposed list, and noted of course that with this type of system there must be a fair chance that the true structure consists of a well-distributed Boltzmann population of a number of almost iso-energetic forms.
Quintuple bonds: part 2
February 20th, 2010In the previous post, I ruminated about how chemists set themselves targets. Thus, having settled on describing regions between two (and sometimes three) atoms as bonds, they added a property of that bond called its order. The race was then on to find molecules which exhibit the highest order between any particular pair of atoms. The record is thus far five (six has been mooted but its a little less certain) for the molecule below
Quintuple bonds
February 16th, 2010Climbers scale Mt. Everest, because its there, and chemists have their own version of this. Ever since G. N. Lewis introduced the concept of the electron-pair bond in 1916, the idea of a bond as having a formal bond-order has been seen as a useful way of thinking about molecules. The initial menagerie of single, double and triple formal bond orders (with a few half sizes) was extended in the 1960s to four, and in 2005 to five. Since then, something of a race has developed to produce the compound with the shortest quintuple bond. One of the candidates for this honour is shown below (2008, DOI: 10.1002/anie.200803859) which is a crystalline species (a few diatomics which exist in the gas phase are also candidates; for other reviews of the topic see 10.1038/nchem.359, 10.1021/ja905035f and 10.1246/cl.2009.1122).
Conformational analysis of cyclotriborazane
February 14th, 2010In an earlier post, I re-visited the conformational analysis of cyclohexane by looking at the vibrations of the entirely planar form (of D6h symmetry). The method also gave interesting results for the larger cyclo-octane ring. How about a larger leap into the unknown?