Posts Tagged ‘Interesting chemistry’
Monday, April 13th, 2009
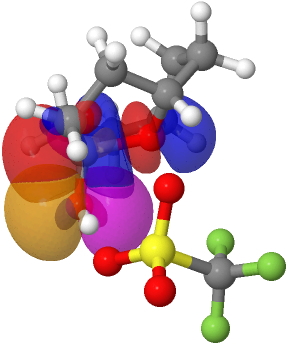
In 1988, Wilke[1] reported molecule 1
![gaytab A [24] annulene. Click on image for model.](http://www.ch.ic.ac.uk/rzepa/blog/wp-content/uploads/2009/04/gaytab.jpg)
A 24-annulene. Click for 3D.
It was a highly unexpected outcome of a nickel-catalyzed reaction and was described as a 24-annulene with an unusual 3D shape. Little attention has been paid to this molecule since its original report, but the focus has now returned! The reason is that a 24- annulene belongs formally to a class of molecule with 4n (n=6) π-electrons, and which makes it
antiaromatic according to the (extended) Hückel rule. This is a select class of molecule, of which the first two members are
cyclobutadiene and cyclo-octatetraene. The first of these is exceptionally reactive and unstable and is the archetypal anti-aromatic molecule. The second is not actually unstable, but it is reactive and conventional wisdom has it that it avoids the undesirable antiaromaticity by adopting a highly non-planar tub shape and hence instead adopts reactive non-aromaticity. Both these examples have localized double bonds, a great contrast with the molecule which sandwiches them, cyclo-hexatriene (i.e. benzene). The reason for the resurgent interest is that a number of crystalline, apparently stable, antiaromatic molecules have recently been discovered, and ostensibly, molecule
1 belongs to this select class!
(more…)
References
- G. Wilke, "Contributions to Organo‐Nickel Chemistry", Angewandte Chemie International Edition in English, vol. 27, pp. 185-206, 1988. https://doi.org/10.1002/anie.198801851
Tags:anti-aromatic systems, chemical shifts, Clar islands, Interesting chemistry, Steve Bachrach, X-ray
Posted in Interesting chemistry | 1 Comment »
Sunday, April 12th, 2009
The diagram below summarizes an interesting result recently reported by Hanson and co-workers (DOI: 10.1021/jo800706y. At ~neutral pH, compound 13 hydrolyses with a half life of 21 minutes, whereas 14 takes 840 minutes. Understanding this difference in reactivity may allow us to understand why some enzymes can catalyze the hydrolysis of peptides with an acceleration of up to twelve orders of magnitude.
(more…)
Tags:chair, conformational analysis, Derek Barton, energy, Hanson, Interesting chemistry, molecular mechanics energy, stable lactone product, stable product
Posted in Interesting chemistry | 1 Comment »
Saturday, April 4th, 2009
Every introductory course or text on aromatic electrophilic substitution contains an explanation along the lines of the resonance diagram shown below. With an o/p directing group such as NH2, it is argued that negative charge accumulates in those positions as a result of the resonance structures shown.
(more…)
Tags:aromatic, Historical, Interesting chemistry, MEP
Posted in Interesting chemistry | No Comments »
Friday, April 3rd, 2009
We recently developed a new computational chemistry practical laboratory here at Imperial College. I gave a talk about it at the recent ACS meeting in Salt Lake City. If you want to see the details of the lab, do go here. The talk itself contains further links and examples. Perhaps here I can quote only the final remark, namely that computational chemistry can now provide chemical accuracy for many problems, including spectroscopy and mechanism, and that the basic tools for doing it can easily be carried around in a backpack! Or, perhaps in the not to distant future, an iPhone!
Tags:ACS, basic tools, chemical accuracy, Chemical IT, computational chemistry, Imperial College, Interesting chemistry, iPhone, Salt Lake City, spectroscopy
Posted in Chemical IT, Interesting chemistry | No Comments »
Thursday, April 2nd, 2009
Mauksch and Tsogoeva have recently published an article illustrating how a thermal electrocyclic reaction can proceed with distoratory ring closure, whilst simultaneously also exhibiting 4n electron Möbius-aromatic character[1]. Why is this remarkable? Because the simple Woodward-Hoffmann rules state that a disrotatory thermal electrocyclic reaction should proceed via a Hückel-aromatic 4n+2 electron transition state. Famously, Woodward and Hoffmann stated there were no exceptions to this rule. Yet here we apparently have one! So what is the more fundamental? The disrotatory character, or the 4n/Möbius character in the example above? Mauksch and Tsogoeva are in no doubt; it is the former that gives, and the latter is correct.
(more…)
References
- M. Mauksch, and S. Tsogoeva, "A Preferred Disrotatory 4<i>n</i> Electron Möbius Aromatic Transition State for a Thermal Electrocyclic Reaction", Angewandte Chemie International Edition, vol. 48, pp. 2959-2963, 2009. https://doi.org/10.1002/anie.200806009
Tags:Hoffmann, Interesting chemistry, jmol, NICS, pericyclic, Reaction Mechanism, Steve Bachrach
Posted in pericyclic | 1 Comment »
Saturday, April 12th, 2008
Tags:animation, Chemical IT, editor, http, Interesting chemistry, jmol, php, Tutorial material, www.ch.imperial.ac.uk/rzepa/blog/wp-content/uploads/2008/04/14-knot.jpg
Posted in Chemical IT, Interesting chemistry | 8 Comments »
![gaytab A [24] annulene. Click on image for model.](http://www.ch.ic.ac.uk/rzepa/blog/wp-content/uploads/2009/04/gaytab.jpg)