In the previous post,[1] I was commenting that the transition state for borohydride reduction of a ketone contained some close contacts between the hydrogen of the borohydride and the hydrogen of water. A systematic search of the CSD reveals a modest number of such contacts have been observed in crystal structures (Table). Since it is always good to have a reality check for constructed transition states, here I take a look at some of compounds showing the closest H…H contacts in the experimental database of structures.
The DFT procedures I used to calculate the geometries of the examples tabled below[2] were
- the classical B3LYP/Def2-TZVPP method, but enhanced with the D4 dispersion correction – the latter developed as a successor to the often used D3+BJ predecessor.
- The DFT method named r2scan-3c, a composite described by its developers as the “Swiss army knife of DFT methods” and “r2SCAN-3c Works Well On Everything”.[3] rather grandly quotesThe specific features are described as “The unaltered r2SCAN functional is combined with a tailor-made triple-ζ Gaussian atomic orbital basis set as well as with refitted D4 and geometrical counter-poise corrections for London-dispersion and basis set superposition error“.[4] The method scales efficiently to several hundred atoms. Results for both types of DFT calculation are collected here.[2]
| Table. Calculated and observed BH…HO interactions | |||||
|---|---|---|---|---|---|
| Molecule | rH…H using B3LYP+D4/ Def2-TZVPP |
rH…H using r2SCAN-3c/ Def2-mTZVPP |
Crystal structure, rH…H, Å |
angle, BH…H, ° | angle, OH…H, ° |
| JATMUN | 1.531 | 1.544 | 1.519 (1.513) [5],[6] |
95.1 | 158.6 |
| OLEVIL | 1.612 | 1.654 | 1.806 (1.67) [7],[8] |
107.7 | 171.4 |
| OLEVEH | 1.623 | 1.666 | 1.857 (1.757) [7],[9] |
105.1 | 176.6 |
| OWUKID | 1.685 | 1.770 | 1.871 (1.778) [10],[11] |
131.7 | 157.8 |
| SASVAS | 1.757 | 1.872 | 1.901 (1.807) [12],[13] |
94.3 | 155.0 |
| BOTFOJ | 1.741 | 1.810 | 1.976 (1.856) [14] |
129.6 | 171.0 |
| MOPXOG | 1.888 | 1.966 | 1.990 (1.872) [15],[16] |
95.4 | 159.5 |
| FOLREF | 1.881 | 1.966 | 1.998 (1.908) [17],[18] |
110.2 | 160.8 |
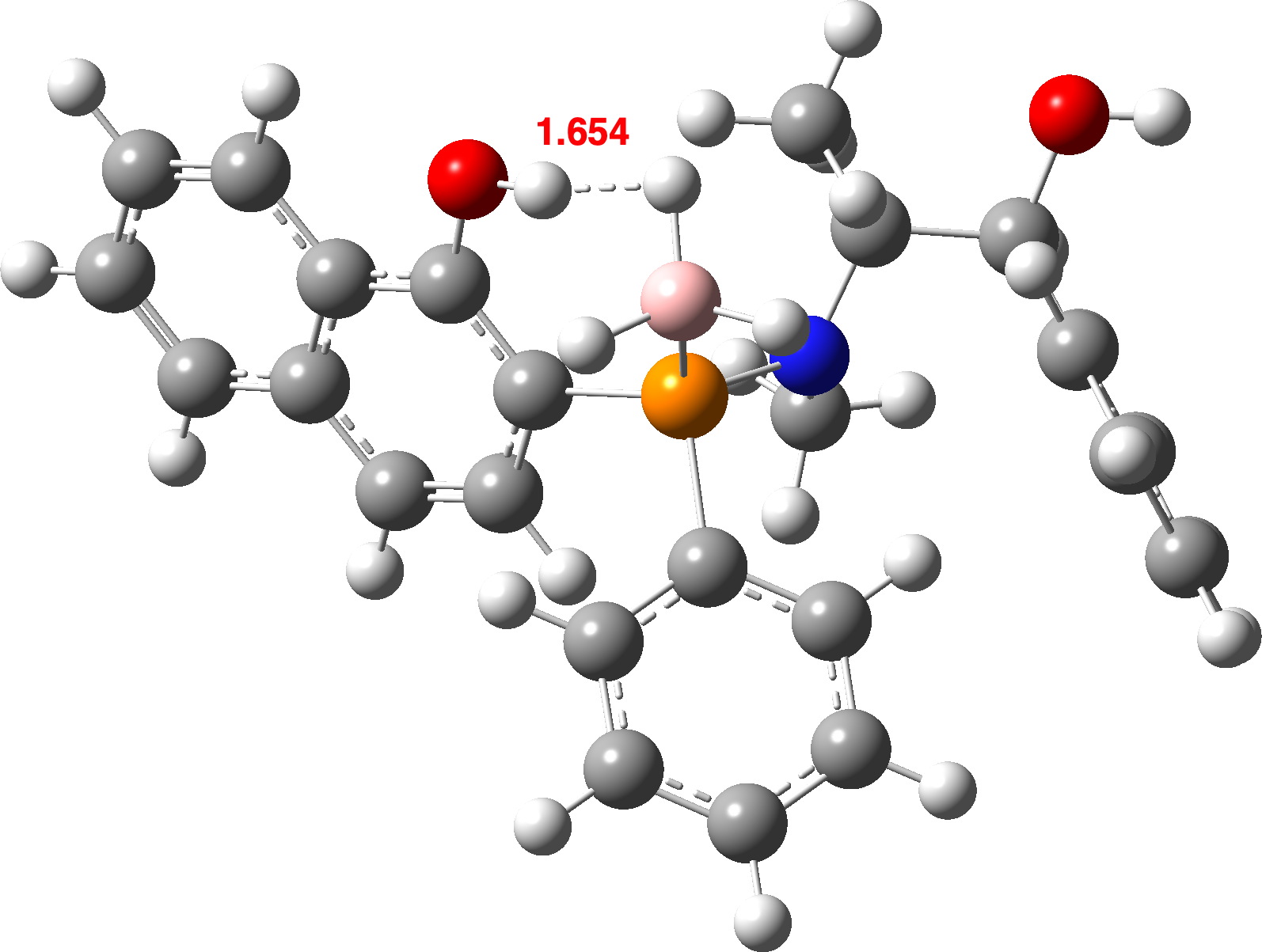
OLEVIL@r2SCAN-3c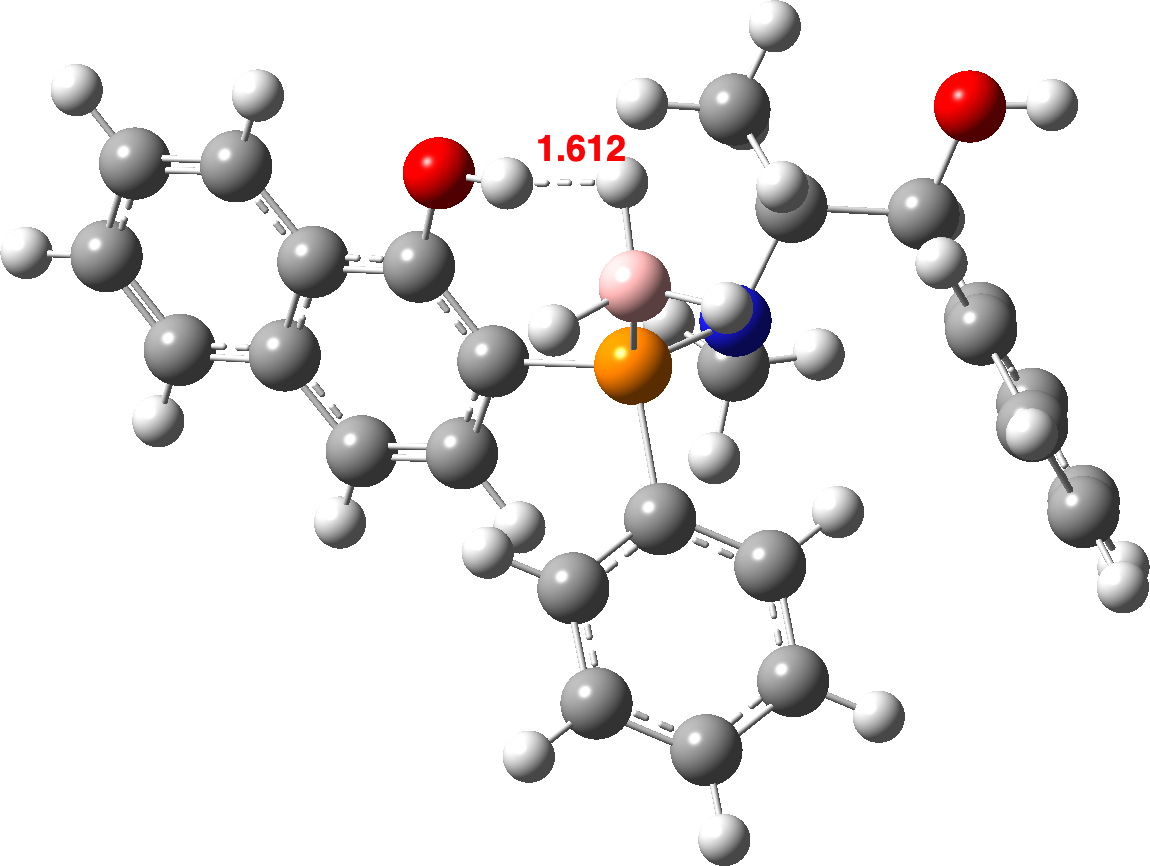
OLEVIL @B3LYP+D4
In general, the B3LYP+D4 method predicts slightly shorter H…H contacts than does r2SCAN-3c. Comparison with experiment is tricky, since OH and BH distances obtained directly from a classical crystal structure refinement tend to emerge as ~0.1A too short (see eg [19] for more detailed discussion). A simple correction for these values is shown in parentheses in the table above. However, given that the angle subtended at the hydrogen atom varies enormously, this correction may too simplistic. Better would be if the original crystallographic data could be re-refined using the non-spherical atom model model described in [19] A provisional conclusion without such treatment might be that r2SCAN-3c is somewhat more accurately predicting the H…H distances.
OLEVIL is interesting because it contains two OH groups, with only one interacting with a proximate BH group. Calculating (r2scan-3c) νOH gives values of 3477 cm-1 as perturbed by the close BH vs a BH unperturbed value of 3801 cm-1 or Δν 324 cm-1. The corresponding values using B3LYP+D4 are 3700 vs 3803, Δν 103 cm-1. This large difference in perturbation predicted by these two DFT methods could be easily tested experimentally. Unfortunately, the experimental information reported for this compound[7] does not contain the OH stretching values, which might have been a good test of the claim that “r2SCAN-3c Works Well On Everything”.
This selection of compounds illustrates how one aspect of a transition state can be given a reality check by comparing a key interaction with experimentally determined crystal structures.
References
- H. Rzepa, "The mechanism of borohydride reductions. Part 2: 4-t-butyl-cyclohexanone – Dispersion induced stereochemistry.", 2025. https://doi.org/10.59350/x5k75-t2m40
- H. Rzepa, "Short B-H...H-O Interactions in crystal structures - a short DFT Exploration using B3LYP+D4 and r2scan-3c", 2025. https://doi.org/10.14469/hpc/15566
- S. Grimme, A. Hansen, S. Ehlert, and J. Mewes, "r2SCAN-3c: An Efficient “Swiss Army Knife” Composite Electronic-Structure Method", 2020. https://doi.org/10.26434/chemrxiv.13333520.v2
- S. Grimme, A. Hansen, S. Ehlert, and J. Mewes, "r2SCAN-3c: A “Swiss army knife” composite electronic-structure method", The Journal of Chemical Physics, vol. 154, 2021. https://doi.org/10.1063/5.0040021
- S. Hoffmann, E. Justus, M. Ratajski, E. Lork, and D. Gabel, "B12H11-containing guanidinium derivatives by reaction of carbodiimides with H3N–B12H11(1−). A new method for connecting boron clusters to organic compounds", Journal of Organometallic Chemistry, vol. 690, pp. 2757-2760, 2005. https://doi.org/10.1016/j.jorganchem.2005.02.037
- Hoffmann, S.., Justus, E.., Ratajski, M.., Lork, E.., and Gabel, D.., "CCDC 252153: Experimental Crystal Structure Determination", 2006. https://doi.org/10.5517/cc8gcz1
- M. Stephan, B. Modec, and B. Mohar, "Asymmetric synthesis of SMS-Phos series’ precursor and a naphthalene analogue", Tetrahedron Letters, vol. 52, pp. 1086-1089, 2011. https://doi.org/10.1016/j.tetlet.2010.12.106
- Stephan, M.., Modec, B.., and Mohar, B.., "CCDC 791282: Experimental Crystal Structure Determination", 2011. https://doi.org/10.5517/ccvkd70
- Stephan, M.., Modec, B.., and Mohar, B.., "CCDC 791281: Experimental Crystal Structure Determination", 2011. https://doi.org/10.5517/ccvkd6z
- A.M.W. Dufter, T.M. Klapötke, M. Rusan, A. Schweiger, and J. Stierstorfer, "Comparison of Functionalized Lithium Dihydrobis(azolyl)borates with Their Corresponding Azolates as Environmentally Friendly Red Pyrotechnic Coloring Agents", ChemPlusChem, vol. 85, pp. 2044-2050, 2020. https://doi.org/10.1002/cplu.202000427
- Dufter, Alicia M. W.., Klapötke, Thomas M.., Rusan, Magdalena., Schweiger, Alexander., and Stierstorfer, Jörg., "CCDC 1998250: Experimental Crystal Structure Determination", 2021. https://doi.org/10.5517/ccdc.csd.cc252bp9
- H. Fernández‐Pérez, S. Donald, I. Munslow, J. Benet‐Buchholz, F. Maseras, and A. Vidal‐Ferran, "Highly Modular POP Ligands for Asymmetric Hydrogenation: Synthesis, Catalytic Activity, and Mechanism", Chemistry – A European Journal, vol. 16, pp. 6495-6508, 2010. https://doi.org/10.1002/chem.200902915
- Fernandez-Perez, H.., Donald, S.M.A.., Munslow, I.J.., Benet-Buchholz, J.., Maseras, F.., and Vidal-Ferran, A.., "CCDC 752671: Experimental Crystal Structure Determination", 2012. https://doi.org/10.5517/cct86qz
- Daniliuc, Constantin G.., Neumann, Markus., Fröhlich, Roland., and Erker, Gerhard., "CCDC 2362258: Experimental Crystal Structure Determination", 2024. https://doi.org/10.5517/ccdc.csd.cc2k93ww
- R. Bigler, E. Otth, and A. Mezzetti, "Chiral Macrocyclic N<sub>2</sub>P<sub>2</sub> Ligands and Iron(II): A Marriage of Interest", Organometallics, vol. 33, pp. 4086-4099, 2014. https://doi.org/10.1021/om5005989
- Bigler, Raphael., Otth, Elisabeth., and Mezzetti, Antonio., "CCDC 1019093: Experimental Crystal Structure Determination", 2014. https://doi.org/10.5517/cc136fzq
- S. Rast, B. Mohar, and M. Stephan, "Efficient Asymmetric Syntheses of 1-Phenyl-phosphindane, Derivatives, and 2- or 3-Oxa Analogues: Mission Accomplished", Organic Letters, vol. 16, pp. 2688-2691, 2014. https://doi.org/10.1021/ol500970x
- Rast, Slavko., Mohar, Barbara., and Stephan, Michel., "CCDC 1007629: Experimental Crystal Structure Determination", 2014. https://doi.org/10.5517/cc12tj5l
- H. Rzepa, "Crystallography meets DFT Quantum modelling.", 2025. https://doi.org/10.59350/5dy8w-0zs92
As always, a great post. Is r2scan3c implemented in any commercial code or did you have to implement it yourself.
Best regards
r2scan-3c is part of ORCA 6.1, which is not commercial. I am not aware whether it has been implemented in any commercial codes (Turbomol might be a likely candidate), but mystery surrounds whether it might be in the forthcoming Gaussian 26, which is supposed to be released this year (i.e. 2025!).
What I can observe is that it runs significantly faster than eg a legacy DFT option such as B3LYP in ORCA. Whether this wil also be true in any other codes it might be implemented in is unknown.
Also unknown at present is which codes Martin Head-Gordon’s new COACH (“Carefully Optimised and Appropriately Constrained Hybrid”) code might appear in. COACH is likely to have similar (improved?) properties to r2scan-3c