The Bürgi–Dunitz angle describes the trajectory of an approaching nucleophile towards the carbon atom of a carbonyl group. A colleague recently came to my office to ask about the inverse, that is what angle would an electrophile approach (an amide)? Thus it might approach either syn or anti with respect to the nitrogen, which is a feature not found with nucleophilic attack. My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder).
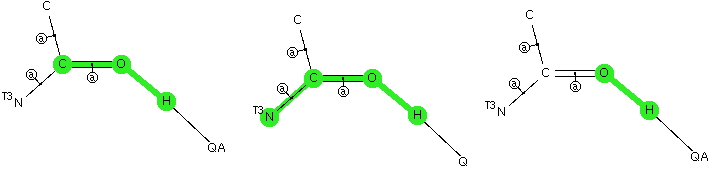 The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl)
The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl) 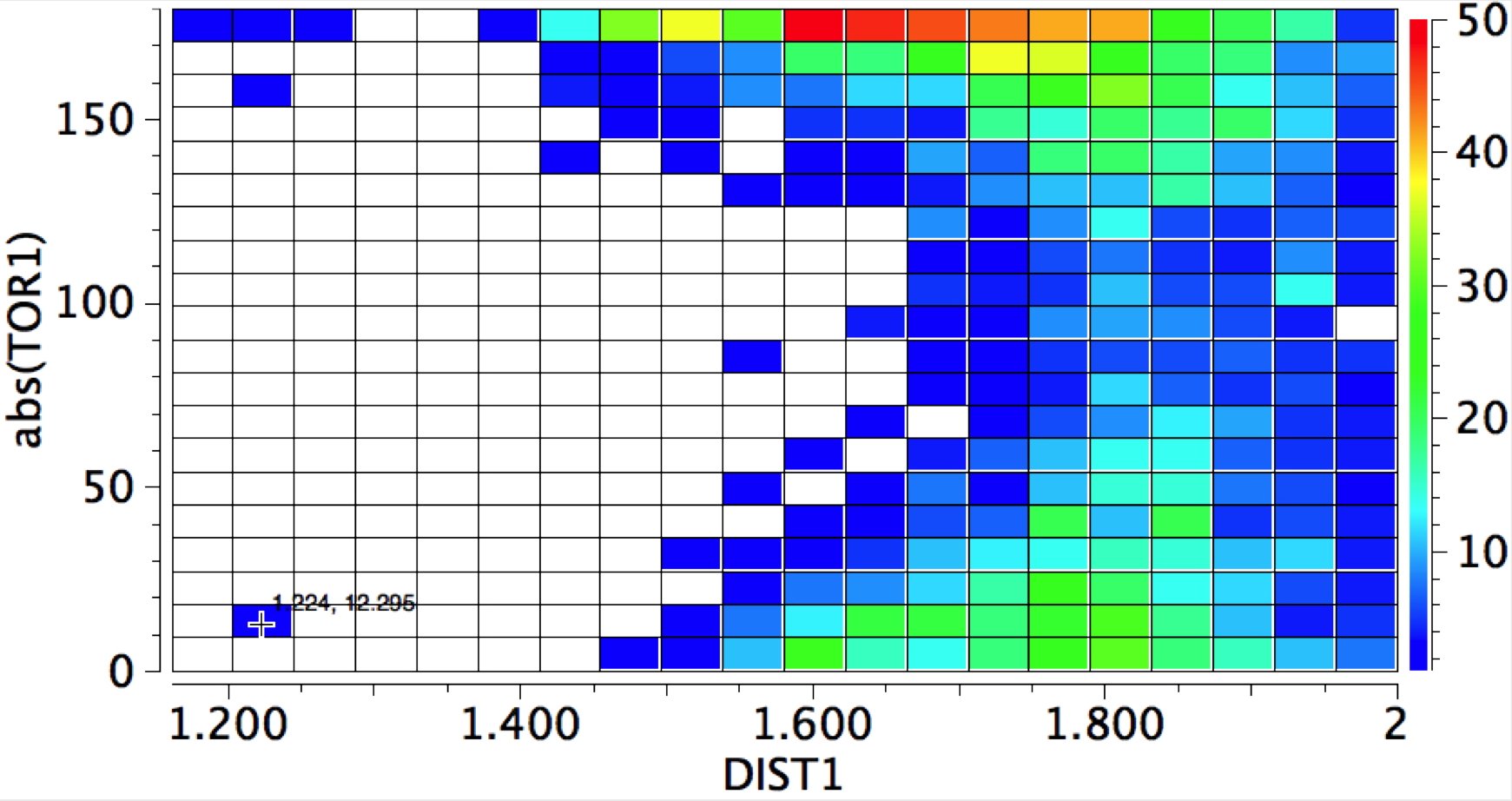
Archive for June, 2014
Anchoring chemistry.
Wednesday, June 18th, 2014I was reminded of this article by Michelle Francl[1], where she poses the question “What anchor values would most benefit students as they seek to hone their chemical intuition?” She gives as common examples: room temperature is 298.17K (actually 300K, but perhaps her climate is warmer than that of the UK!), the length of a carbon-carbon single bond, the atomic masses of the more common elements.
References
- M. Francl, "Take a number", Nature Chemistry, vol. 5, pp. 725-726, 2013. https://doi.org/10.1038/nchem.1733
Test of JSmol in WordPress: the background story.
Sunday, June 8th, 2014A word of explanation about this test page for experimenting with JSmol. Many moons ago I posted about how to include a generated 3D molecular model in a blog post, and have used that method on many posts here ever since. It relied on Java as the underlying software (first introduced in 1996), or almost 20 years ago. Like most software technologies, much has changed, and Java itself (as a compiled language) has had to move to improve its underlying security. In the last year, the Java code itself (in this case Jmol) has needed to be digitally signed in a standard manner, and this meant that many an old site that used unsigned older versions has started to throw up increasingly alarming messages.
Kekulé’s vibration: A modern example of its use.
Friday, June 6th, 2014Following the discussion here of Kekulé’s suggestion of what we now call a vibrational mode (and which in fact now bears his name), I thought I might apply the concept to a recent molecule known as [2.2]paracyclophane. The idea was sparked by Steve Bachrach’s latest post, where the “zero-point” structure of the molecule has recently been clarified as having D2 symmetry.[1]
References
- H. Wolf, D. Leusser, M. Jørgensen, R. Herbst‐Irmer, Y. Chen, E. Scheidt, W. Scherer, B.B. Iversen, and D. Stalke, "Phase Transition of [2,2]‐Paracyclophane – An End to an Apparently Endless Story", Chemistry – A European Journal, vol. 20, pp. 7048-7053, 2014. https://doi.org/10.1002/chem.201304972