Archive for July, 2014
Saturday, July 19th, 2014
Whilst clusters of carbon atoms are well-known, my eye was caught by a recent article describing the detection of a cluster of boron atoms, B40 to be specific.[1] My interest was in how the σ and π-electrons were partitioned. In a C40, one can reliably predict that each carbon would contribute precisely one π-electron. But boron, being more electropositive, does not always play like that. Having one electron less per atom, one might imagine that a fullerene-like boron cluster would have no π-electrons. But the element has a propensity[2] to promote its σ-electrons into the π-manifold, leaving a σ-hole. So how many π-electrons does B40 have? These sorts of clusters are difficult to build using regular structure editors, and so coordinates are essential. The starting point for a set of coordinates with which to compute a wavefunction was the supporting information. Here is the relevant page: 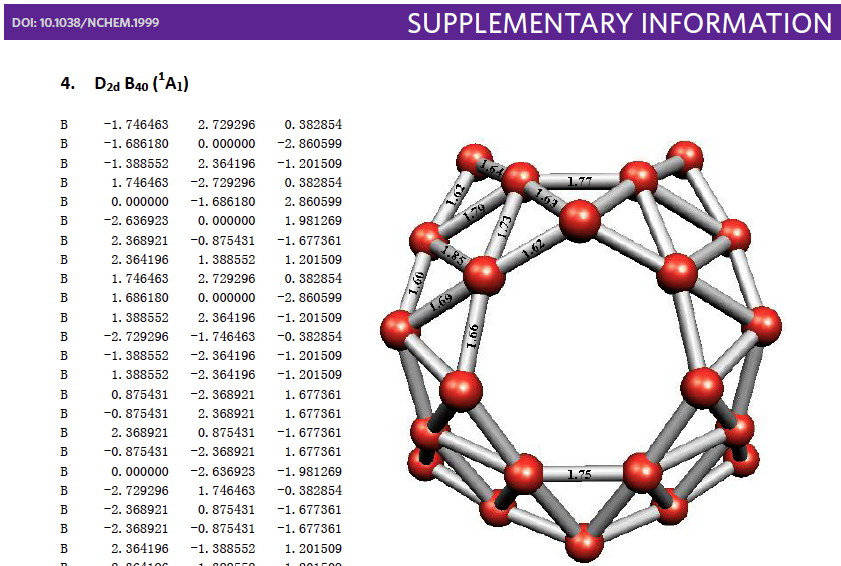 The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
(more…)
References
- H. Zhai, Y. Zhao, W. Li, Q. Chen, H. Bai, H. Hu, Z.A. Piazza, W. Tian, H. Lu, Y. Wu, Y. Mu, G. Wei, Z. Liu, J. Li, S. Li, and L. Wang, "Observation of an all-boron fullerene", Nature Chemistry, vol. 6, pp. 727-731, 2014. https://doi.org/10.1038/nchem.1999
- H.S. Rzepa, "The distortivity of π-electrons in conjugated boron rings", Physical Chemistry Chemical Physics, vol. 11, pp. 10042, 2009. https://doi.org/10.1039/b911817a
Tags:Acrobat, Adobe, chemical markup, DOS, operating systems, PDF, pence, Unix
Posted in Chemical IT, Interesting chemistry | 2 Comments »
Saturday, July 12th, 2014
Computational quantum chemistry has made fantastic strides in the last 30 years. Often deep insight into all sorts of questions regarding reactions and structures of molecules has become possible. But sometimes the simplest of questions can prove incredibly difficult to answer. One such is how accurately can the boiling point of water be predicted from first principles? Or its melting point? Another classic case is why mercury is a liquid at room temperatures? The answer to that question (along with another, why is gold the colour it is?) is often anecdotally attributed to Einstein. More accurately, to his special theory of relativity.[1] But finally in 2013 a computational proof of this was demonstrated for mercury.[2] The proof was built up in three stages.
(more…)
References
- A. Einstein, "Ist die Trägheit eines Körpers von seinem Energieinhalt abhängig?", Annalen der Physik, vol. 323, pp. 639-641, 1905. https://doi.org/10.1002/andp.19053231314
- F. Calvo, E. Pahl, M. Wormit, and P. Schwerdtfeger, "Evidence for Low‐Temperature Melting of Mercury owing to Relativity", Angewandte Chemie International Edition, vol. 52, pp. 7583-7585, 2013. https://doi.org/10.1002/anie.201302742
Tags:bulk metal, pence, potential energy surfaces
Posted in General | 4 Comments »
Saturday, July 5th, 2014
I was lucky enough to attend the announcement made in 2012 of the discovery of the Higgs Boson. It consisted of a hour-long talk mostly about statistics, and how the particle physics community can only claim a discovery when their data has achieved a 5σ confidence level. This represents a 1 in 3.5 million probability of the result occurring by chance. I started thinking: how much chemistry is asserted at that level of confidence? Today, I read Steve Bachrach’s post on the structure of Citrinalin B and how “use of Goodman’s DP4 method indicates a 100% probability that the structure of citrinalin B is (the structure below)”. Wow, that is even higher than the physicists. Of course, 100% has been obtained by rounding 99.7 (3σ is 99.73%) or whatever (this is one number that should never be so rounded!). 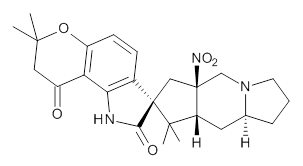 But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
(more…)
References
- E.V. Mercado-Marin, P. Garcia-Reynaga, S. Romminger, E.F. Pimenta, D.K. Romney, M.W. Lodewyk, D.E. Williams, R.J. Andersen, S.J. Miller, D.J. Tantillo, R.G.S. Berlinck, and R. Sarpong, "Total synthesis and isolation of citrinalin and cyclopiamine congeners", Nature, vol. 509, pp. 318-324, 2014. https://doi.org/10.1038/nature13273
- T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao, and J. Kobayashi, "Absolute Stereochemistry of Citrinadins A and B from Marine-Derived Fungus", The Journal of Organic Chemistry, vol. 70, pp. 9430-9435, 2005. https://doi.org/10.1021/jo051499o
- F. Cherblanc, Y. Lo, E. De Gussem, L. Alcazar‐Fuoli, E. Bignell, Y. He, N. Chapman‐Rothe, P. Bultinck, W.A. Herrebout, R. Brown, H.S. Rzepa, and M.J. Fuchter, "On the Determination of the Stereochemistry of Semisynthetic Natural Product Analogues using Chiroptical Spectroscopy: Desulfurization of Epidithiodioxopiperazine Fungal Metabolites", Chemistry – A European Journal, vol. 17, pp. 11868-11875, 2011. https://doi.org/10.1002/chem.201101129
- F.L. Cherblanc, Y. Lo, W.A. Herrebout, P. Bultinck, H.S. Rzepa, and M.J. Fuchter, "Mechanistic and Chiroptical Studies on the Desulfurization of Epidithiodioxopiperazines Reveal Universal Retention of Configuration at the Bridgehead Carbon Atoms", The Journal of Organic Chemistry, vol. 78, pp. 11646-11655, 2013. https://doi.org/10.1021/jo401316a
Tags:chemical, Reading, Steve Bachrach, X-ray
Posted in General | 2 Comments »
Thursday, July 3rd, 2014
Increasingly, our access to scientific information is becoming a research topic in itself. Thus an analysis of big deal journal bundles[1] has attracted much interesting commentary (including one from a large scientific publisher[2]). In the UK, our funding councils have been pro-active in promoting the so-called GOLD publishing model, where the authors (aided by grants from their own institution or others) pay the perpetual up-front publication costs (more precisely the costs demanded by the publishers, which is not necessarily the same thing) so that their article is removed from the normal subscription pay wall erected by the publisher and becomes accessible to anyone. As the proportion of GOLD content increases, it was anticipated (hoped?) that the costs of accessing the remaining non-GOLD articles via a pay-walled subscription would decrease.
(more…)
References
- T.C. Bergstrom, P.N. Courant, R.P. McAfee, and M.A. Williams, "Evaluating big deal journal bundles", Proceedings of the National Academy of Sciences, vol. 111, pp. 9425-9430, 2014. https://doi.org/10.1073/pnas.1403006111
- C. Woolston, "Secret publishing deals exposed", Nature, vol. 510, pp. 447-447, 2014. https://doi.org/10.1038/510447f
Tags:GBP, typical article processing charge ranges, United Kingdom
Posted in Chemical IT, General | 8 Comments »
 The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable. But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes