|
|
|
|
Cubane, a landmark in the world of "impossible" compounds, has been found to have a rich chemistry, full of the unexpected. The recent renaissance of cubane chemistry, triggered by potential applications of the system to the production of high-energy fuels and propellants, has lead to many discoveries including the first methods for systematic subsitiution on strained, saturated systems and a new process for the metalation of arenes, ortho magnesation. Reactive intermediates with exceptional bonding parameters have been uncovered and characterised including
and many other extraordinary species.
Cubane is an immensely strained molecule; the geometry an each carbon is far from the tetrahedral angle. Originally there was doubt that the skeleton could even hold together. Cubane is shown below:
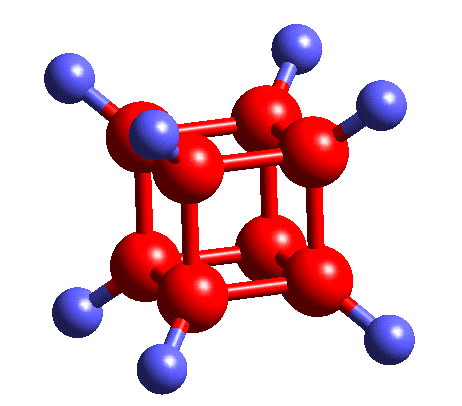
The fascination with cubane chemistry has continued unabated ever since the maiden construction of the cubane nucleus by Eaton and Cole in 1964. From a brief search of the cubane literature it is obvious that the pioneer in its synthesis and further substitution was Philip E. Eaton.
Cubanes have found applications in diverse areas of science, including:
It has even been hypothesised that the cubane analogue octaazacubane (N8), if formed would be relatively stable. While it is proposed to be highly unstable thermodynamically, its decomposition to N2 is forbidden by orbital symmetry, in the same way as for cubane.
|
|
|