|
|
|
|
Cubane is a saturated hydrocarbon of extraordinary geometry and strain. Although it was once dismissed as impossible, it has been shown to be stable. Similarly, the cubyl cation has been found to be far more readily accessible than once imagined. With these lessons in mind, it is prudent not to dismiss other uncommon notions out of hand. The following topics are covered briefly here:
Ring Opening Reactions of Cubanes
Cubane and many substituted cubanes are very stable compounds, indeed remarkably so considering the extreme strain energy of the skeleton (see Properties). There is no symmetry-allowed, concerted pathway for ring opening. When reagents or substituents open other paths, the molecule rearranges.
Here we will just consider electron-rich cubanes. Although cubanols can be isolated with care, they are fragile. 4-Methylcubanol, like the others, opens by way of homoketonization to a tricyclooctenone, which in turn rearranges further to a vinylcyclobutenylketene. The scheme is given below:
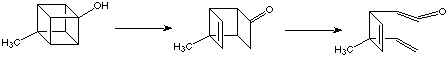
Cubylamines show similar ring-opening proclivities, but their ammonium salts are quite stable. Clearly, the ring-opening process is initiated by the shift of electrons into the ring. The tendency toward ring opening is greater when there is an electron acceptor at C2. No cubylamine or cubanol with a carbonyl substituent ortho to the electron donor has ever been obtained. Similarly, 2-nitrocubylamine has never been isolated. A simple push-pull mechanism can easily be rationalise the rapid bond cleavages which account for the instability of such compounds.
1(9) - Homocubenes
K.-L. Hoffmann in my group showed that cubyl phenyl diazomethane on thermolysis or photolysis is converted to 9-phenyl-1(9)-homocubene:
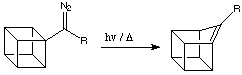
It was later shown that the same reaction occurs when R = H.
The 1(9)-homocubene system contains the most twisted C-C double bond known. If rehybridisation and pyramidalization did not occur the angle between the p orbitals of the unsaturated carbons would be 90o. This is the anti-Bredt olefin nonpareil! Its chemistry is extraordinary. 1(9)-homocubenes rearrange to 9-homocubylidenes. These conversions are amongst the rare examples of the rearrangement of an olefin to a carbene.
1,2-Dehydrocubane (Cubene)
Cubene is the most extreme example of a pyramidalized olefin. Obviously, the geometry about the vinyl carbon atom can be nowhere near planar. The pyramidalization angle has been calculated to be 84o.
In the original experimental work cubene was generated by reaction of 1,2-diiodocubane with an organolithium:
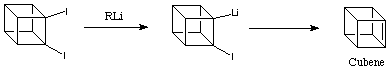
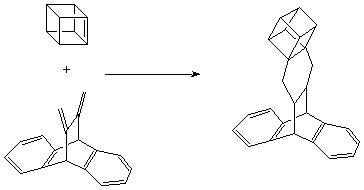
Cubene is an immensely strained, relatively "open", and sterically unhindered olefin. As such, it can be expected to be very reactive in Diels-Alder additions. Indeed, when the deiodination of 1,2-diiodocubane was conducted at room temperature with tert -butyllithium in benzene in the presence of 11,12-dimethylene-9,10-dihydro-9,10-ethanoanthracene, the 1:1 Diels-Alder adduct was formed in 64% isolated yield. Shown below:
Octaazacubane (N8)
The cubane analogue octaazacubane is a metastable molecule constructed entirely of nitrogen atoms. It is shown below:
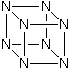
While one would expect this molecule to be highly unstable thermodynamically, pathways for decomposition to ground state N2 are forbidden by orbital symmetry. This suggests that octaazacubane would have a significant barrier to decomposition. These orbital symmetry rules are identical with those for cubane, which as we know has been synthesised and well characterised. One therefore would expect that N8, if formed, would be relatively stable!
|
|
|