|
|
|
|
The first synthesis of the Cubane structure was successfully carried out in 1964 by Philip E. Eaton and Thomas W. Cole in the Department of Chemistry at the University of Chicago in Illinois. This is shown below:
Philip E. Eaton Synthesis:
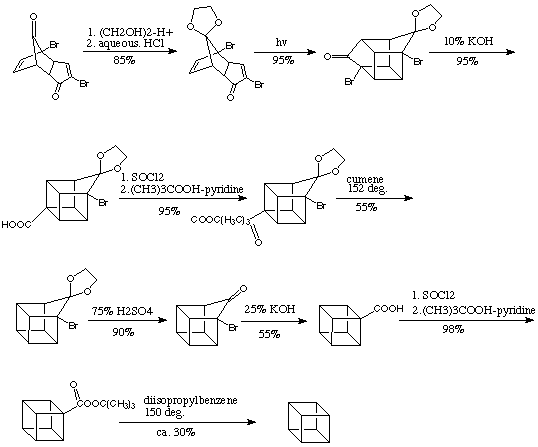
Subsequential this was improved a few years later by N.B. Chapman et al. in England, and the cubane synthesis ultimately evolved into a simple five-step process providing cubane-1,4-dicarboxylic acid in about 25% overall yield.
Improved Synthesis:
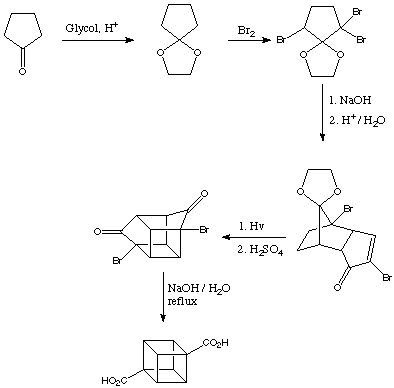
The carboxylic acid groups on cubane-1,4-dicarboxylic acid turned out to be exceedingly well behaved. For information regarding possible interconversions of the carboxylic group, see Functional Group Transformations.
An alternative synthesis was proposed by James C. Barborak, L. Watts and R. Pettit in the Department of Chemistry, University of Texas, in 1966. They noted from initial experiments that the organometallic complex cyclobutadiene-iron tricarbonyl seemed to afford a useful source of the normally unstable cyclobutadiene. During the oxidative decomposition of the iron complex with ceric ion in the presence of dienophiles a molecule of cyclobutadiene can be transferred form the iron atom to the dienophile. In order to demonstrate the general utility of the reaction they proposed the synthesis of the cubane system, in which the cyclobutadiene transfer reaction plays a key role. The reaction scheme is shown below:
James C. Barborak Synthesis:
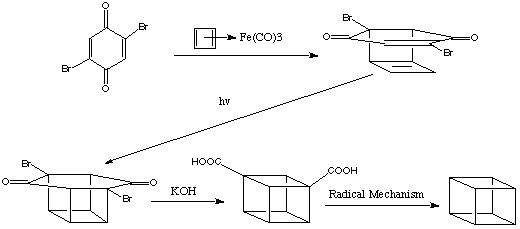
The dibromodiketone was converted to cubane-1,4-dicarboxylic acid by the Favorskii rearrangement. Decarboxylation of cubane-1,4-dicarboxylic acid was carried out via thermal degradation of the di-t -butyl perester by heating in isopropyl benzene. It should be noted that the product of the initial reaction of the 2,5-dibromo-1,4-benzo quinone is the endo isomer due to maximum orbital overlap.
Improved Decarboxylation Method:
The original decarboxylation method via the tert -butyl perester has been superseded by a far superior pathway. This is shown below:
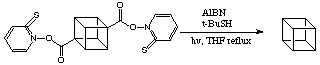
Availability of Cubanes.
The synthesis of cubane-1,4-dicarboxylic acid has been scaled up and is now conducted in small pilot plants by Fluorochem in California and EniChem Synthesis in Milan. It is now being made in multi-kilogram batches. Nevertheless, cubane and its derivatives are still expensive to purchase.
Cubane the hydrocarbon can be easily obtained by the above improved decarboxylation on a 10-gram scale in very nearly quantitative yield. It is beautifully crystalline; alas, the crystals are rhombohedral, not cubic.
|
|