Here we make no attempt to describe the rich variety of inorganic reactivity that can also be described as pericyclic. There are four major organic categories covering a very wide scope of organic and organometallic chemistry, and which form a rich basis for understanding the more subtle aspects of synthesis and organic chemistry.
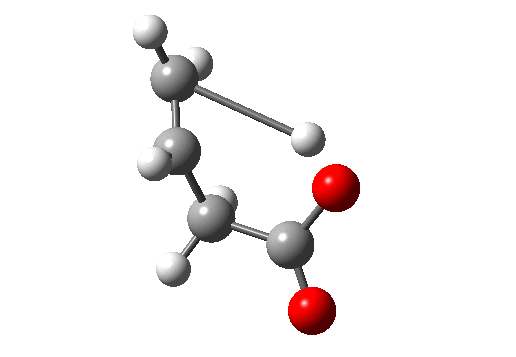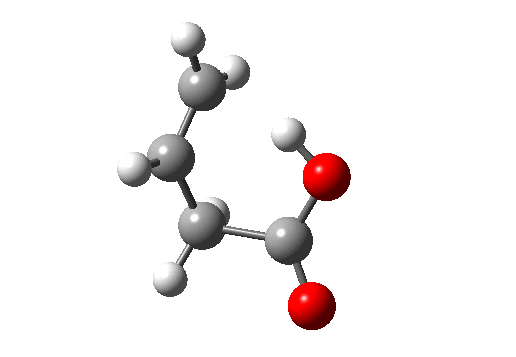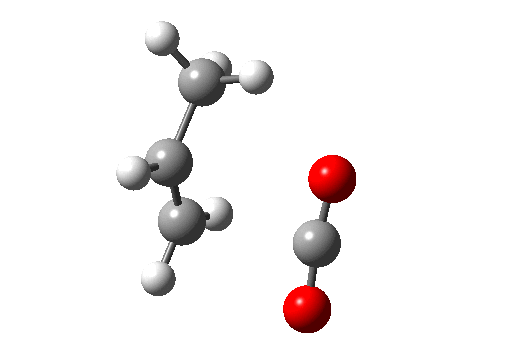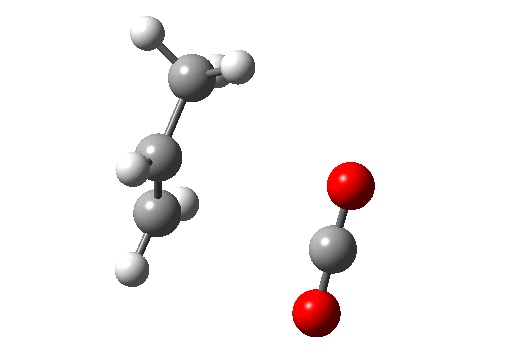
Before dealing with real pericyclic reactions, we should exclude bond-shifting isomerisations (as below, a relatively slow process, ~ms) or resonance (as in benzene, an extremely fast process, ~fs). At least one σ bond has to make or break for a pericyclic reaction proper.
An electrocyclic reaction involves the concerted formation of one σ bond between the two ends of a linear conjugated π system, or the reverse reaction in which one σ bond is broken to produce a linear conjugated system. In these definitions, by 'conjugated', we mean a system of C-C multiple bonds, or of lone pairs.
 |
 |
A cycloaddition reaction involves the concerted formation of two or more σ bonds between the termini of two or more conjugated π systems. The reverse reaction involves the concerted cleavage of two or more σ bonds to produced two or more conjugated π systems.
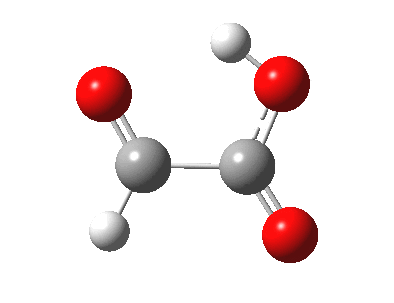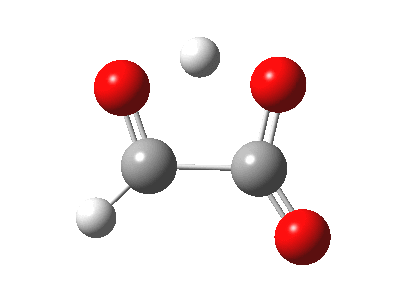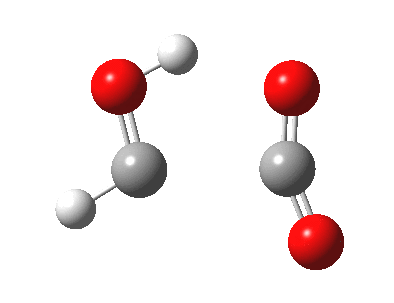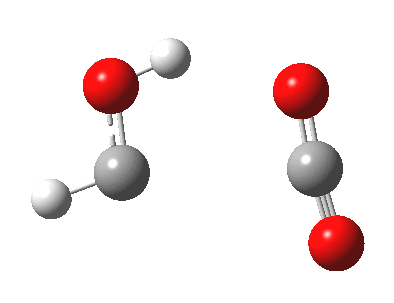
The last example above is a three-component π2s + π2s + π2s cyclo-elimination reaction. For the specific case where formation or cleavage of two σ bonds occurs at a single atom, the reaction is called a Cheletropic reaction.
A sigmatropic reaction involves the concerted migration of an atom or group from one point of attachment to a conjugated system to another point of attachment, during which one σ bond is broken and one σ bond is made. A sigmatropic reaction can be classified according to the length of the group that migrates, and the length of the backbone along which it migrates;
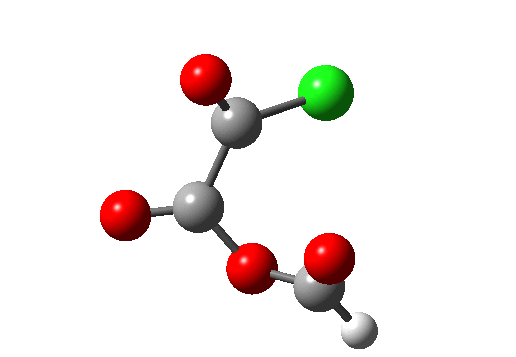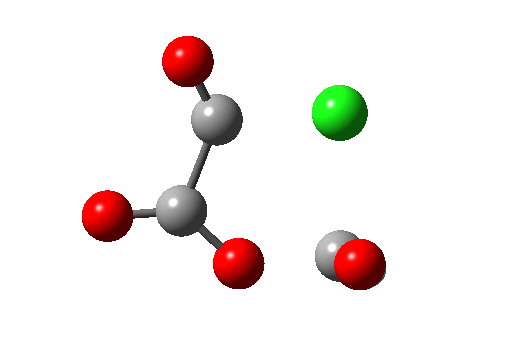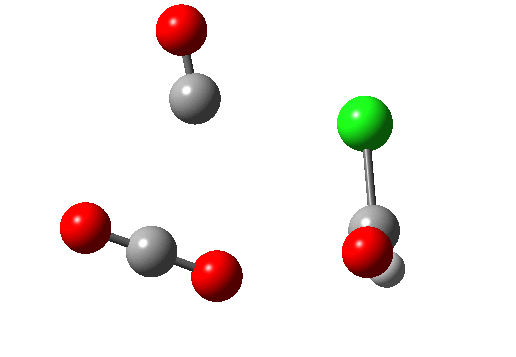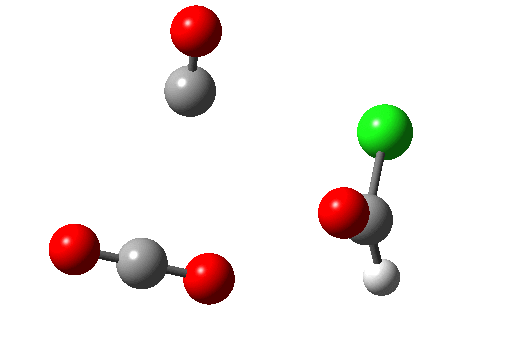
A general category which involves the formation and cleavage of unequal numbers of σ bonds in a concerted cyclic transition state. The top right example shows part of the mechanism for transforming a carboxylic acid to an acyl chloride using oxalyl chloride (three bonds broken, one made). This reaction also has features of a cheletropic elimination (above). The last example (bottom right) is of some recent interest (DOI: 10.1038/nature07010), involving the preparation of hydroxycarbene and its remarkable stabilization by deuterium isotope effect inhibition of the hydrogen tunnelling that is thought to be responsible for the very short lifetime of the undeuterated species (2 hours at 11K for the hydrogen version, 2000 years for the deuterium, an isotope effect of about 9 million!).
All these examples are 6-electron/4n+2 thermal processes.
 |
 |
 |
|
 A newly identified type of pericyclic reaction is the so-called bispericyclic, which simultaneously sustains the characteristics of two or more separate pericyclic reactions. Originally reported as the mechanism for the dimerisation of cyclopentadiene, it is now thought to be more common than previously thought. This has simultaneous characteristics of both a π2s + π4s and a π4s + π2s cycloaddition (they are not the same!) as well as a [3,3] sigmatropic shift. You will encounter this reaction in the third year molecular modelling lab
A newly identified type of pericyclic reaction is the so-called bispericyclic, which simultaneously sustains the characteristics of two or more separate pericyclic reactions. Originally reported as the mechanism for the dimerisation of cyclopentadiene, it is now thought to be more common than previously thought. This has simultaneous characteristics of both a π2s + π4s and a π4s + π2s cycloaddition (they are not the same!) as well as a [3,3] sigmatropic shift. You will encounter this reaction in the third year molecular modelling lab
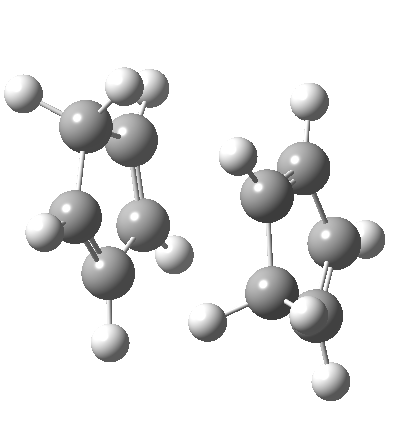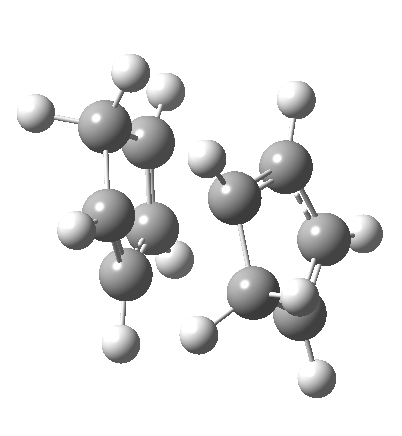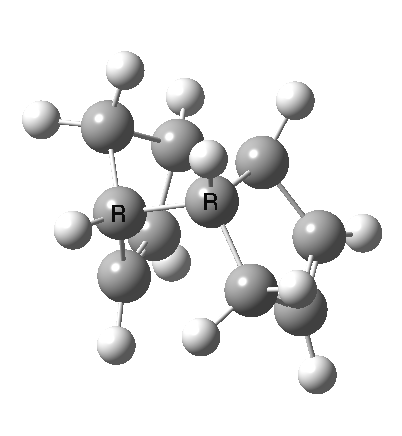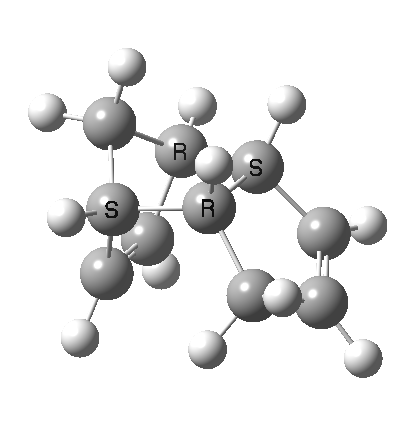 |
Another pericyclic type with multiple-personalities involves cyclopropane rings, which are often regarded as honorary alkenes. Should this be regarded as closer to an electrocyclic reaction of a triene (as above, with the cyclopropane acting an an alkene) or as a [1,5] sigmatropic rection (with the cyclopropane acting as a single bond)? This can also be regarded as a manifestation of homo-aromatic transition states (see later).
© Henry S. Rzepa, 1978-2014. Hide|show Toolbar.