| [Related articles/posters: None: require molecules] |
Activation of the
interelement ![]() -bonds
by transition metal complexes has recently become a subject of growing reserch
interest.[1-4] We have studied palladium-catalyzed
bis-silylation reactions, which have successfully led to the stereoselective
synthesis of polyols,[5]
optically active allylsilanes,[6]
and allenylsilanes.[7]
-bonds
by transition metal complexes has recently become a subject of growing reserch
interest.[1-4] We have studied palladium-catalyzed
bis-silylation reactions, which have successfully led to the stereoselective
synthesis of polyols,[5]
optically active allylsilanes,[6]
and allenylsilanes.[7]
In this paper, we desclose palladium- and platinum-catalyzed silaboration of carbon-carbon multiple bonds through activation of silicon-boron bond of silylboranes.[8]
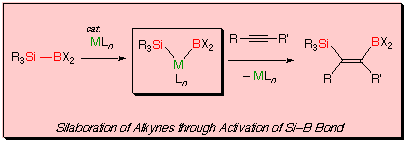
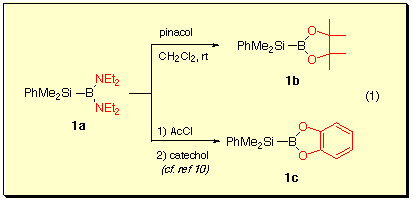
Silylborane 1a was prepared by the method reported by Nšth et al.[9] and 1b and 1c were obtained by ligand exchange reaction of 1a.[10]
Reactions of silylboranes 1a-c and 1-octyne were examined in the presence of various transition metal catalysts.
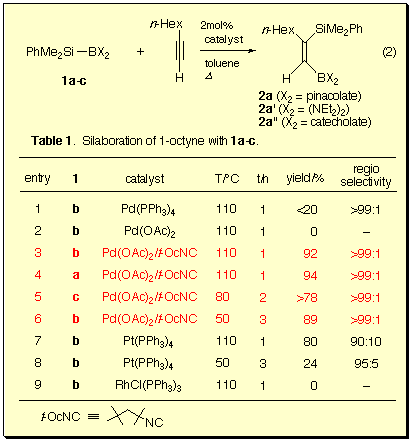
We found that 1b was the silylborane of choice for the present silaboration reaction with respect to the stability of the product during isolation. Furthermore, the isonitrile-palladium(0) catalyst was most effective and provided (Z)-1-boryl-2-silyloct-1-ene with perfect regio- and stereoselectivity, which were confirmed by NOE experiments.
Various terminal alkynes underwent the silaboration regio- and stereoselectively in the presence of the catalyst.
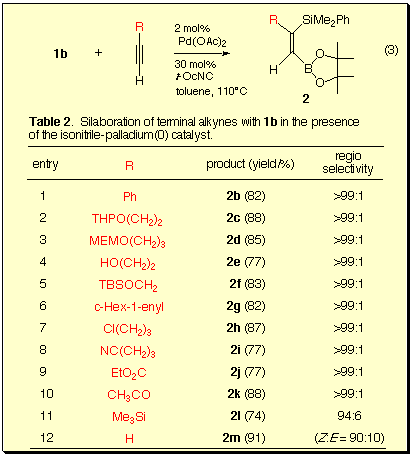
The silaboration was also applicable to phenyl-substituted internal alkynes. In the case of 1-phenylpropyne, regioselective addition of the silicon-boron bond took place to give product having silyl group at the 1-position.
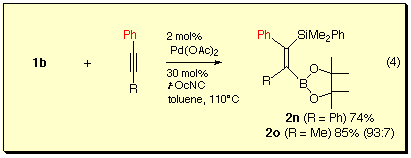
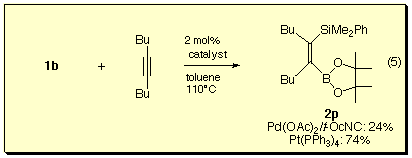
For dialkyl-substituted alkyne, platinum-catalyst gave silaboration product in much better yield than the palladium catalyst.
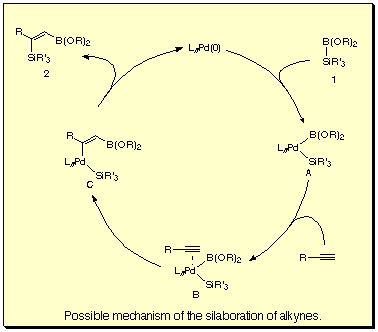
A possible mechanism is shown in the following scheme.
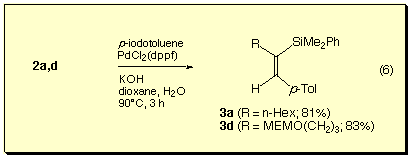
The silaboration products thus obtained underwent cross-coupling reactions with iodotoluene in the presence of a palladium catalyst.[11] The reaction occurred only at the boryl group to give (Z)-beta-silylstyrene derivatives in good yields.
References and Notes
[1] For silicon-silicon bonds, see: (a) K. A. Horn, Chem. Rev. 1995, 95, 1317. (b) H. K. Sharma, K. H. Pannell, Chem. Rev. 1995, 95, 1351.
[2] For silicon-tin bonds, see: (a) T. N. Mitchell, H. Killing, R. Dicke, R. Wickenkamp, J. Chem. Soc., Chem. Commun. 1985, 354. (b) B. L. Chenard, C. M. V. Zyl, J. Org. Chem. 1986, 51, 3561. (c) M. Murakami, H. Amii, N. Takizawa, Y. Ito, Organometallics 1993, 12, 4223.
[3] For boron-boron bonds, see: (a) T. Ishiyama, N. Matsuda, N. Miyaura, A. Suzuki, J. Am. Chem. Soc. 1993, 115, 11018. (b) T. Ishiyama, N. Matsuda, M. Murata, F. Ozawa, A. Suzuki, N. Miyaura, Organometallics 1996, 15, 713Đ720. (c) R. T. Baker, P. Nguyen, T. B. Marder, S. A. Westcott, Angew. Chem. 1995, 107, 1451. Angew. Chem., Int. Ed. Engl. 1995, 34, 1336. (d) T. Ishiyama, M. Yamamoto, N. Miyaura, J. Chem. Soc., Chem. Commun. 1997, 689.
[4] For boron-tin bonds, see: S. Onozawa, Y. Hatanaka, T. Sakakura, S. Shimada, M. Tanaka, Organometallics 1996, 15, 5450.
[5] (a) M. Murakami, M. Suginome, K. Fujimoto, H. Nakamura, P. G. Andersson, Y. Ito, J. Am. Chem. Soc. 1993, 115, 6487. (b) M. Suginome, A. Matsumoto, K. Nagata, Y. Ito, J. Organomet. Chem. 1995, 499, C1. (c) M. Suginome, Y. Yamamoto, K. Fujii, Y. Ito, J. Am. Chem. Soc. 1995, 117, 9608.
[6] (a) M. Suginome, A. Matsumoto, Y. Ito, J. Am. Chem. Soc. 1995, 118, 3061. (b) M. Suginome, T. Iwanami, A. Matsumoto, Y. Ito, Tetrahedron: Asym. 1997, 859.
[7] M. Suginome, A. Matsumoto, Y. Ito, J. Org. Chem. 1996, 61, 4884.
[8] A part of this paper was reported preliminarily. M. Suginome, H. Nakamura, Y. Ito, J. Chem. Soc., Chem. Commun. 1996, 2777.
[9] W. Biffar, H. Nšth, R. Schwerthšffer, Liebigs Ann. Chem. 1981, 2067.
[10] J. D. Buynak, B. Geng, Organometallics 1995, 14, 3112.
[11] N. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457.
#Top