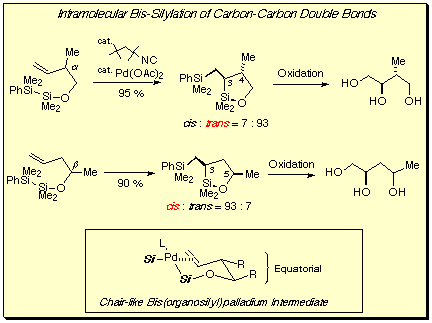
Intramolecular bis-silylation of homoallylic alcohols proceeded with 5-exo cyclization in the presence of isonitrile-palladium(0) catalyst.[5a] The bis-silylation took place highly stereoselectively: an alkene having a substituent alpha to the C=C bond afforded trans-3,4-substituted oxasilolane and that having a beta substituent gave cis-3,5-substituted oxasilolane. The 1,2-oxasilolanes thus produced stereoselectively were oxidatively converted to the corresponding 1,2,4-triols by Tamao oxidation.

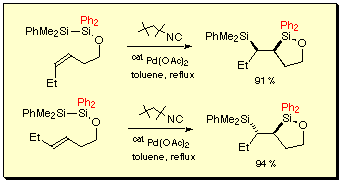
The bis-silylation was applicable not only for terminal alkenes, but also geminally- and vicinally-disubstituted alkenes. In the latter case, use of 1,1-diphenyl-substituted disilanyl groups was essential to obtain bis-silylation products in good yields.[5b]
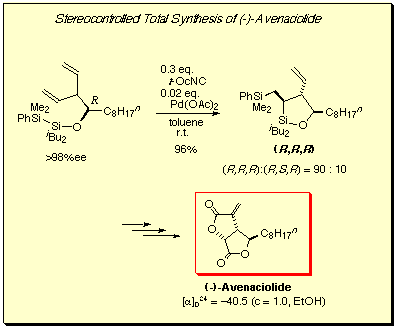
(-)-Avenaciolide, an antifungal metabolite, was synthesized through intramolecular bis-silylation of optically pure 3-vinyldodecen-4-ol, which produced one of four diastereomers stereoselectively.[5c]