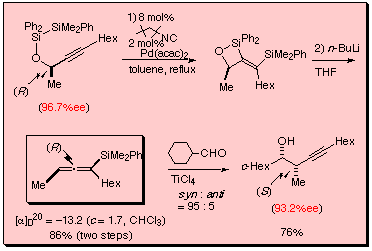
We have reported the synthesis of chiral allenylsilanes through intramolecular bis-silylation of disilanyl ethers of chiral propargylic alcohols. The intramolecular bis-silylation proceeded in the presence of a isonitrile-palladium(0) catalyst prepared from Pd(acac)2 (2 mol%) and 1,1,3,3-tetramethylbutyl isocyanide (8 mol%) to give four-membered products nearly quantitatively. The bis-silylation products were directly treated with n-BuLi to give allenylsilanes in good yields via Peterson-type elimination. A highly enantio-enriched allenylsilane was synthesized from (R)-3-decyn-2-ol with 96.7 %ee by the bis-silylation followed by the treatment with n-BuLi. Subsequent TiCl4-mediated reaction of the allenylsilane with cyclohexanecarboxyaldehyde proceeded highly stereoselectively to give syn-homopropargylic alcohol with 93.2 %ee.
