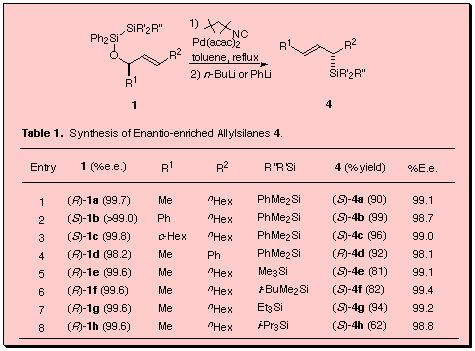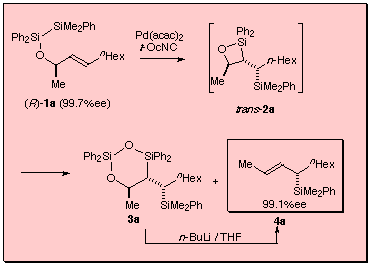
We have recently reported that highly enantio-enriched allylsilanes were synthesized by isonitrile-palladium(0) catalyzed intramolecular bis-silylation of chiral allyl alcohols and subsequent Peterson-type elimination with organolithium reagents. The intramolecular bis-silylation may proceed via highly stereoselective formation of trans-substituted silaoxetane, which undergoes disproportionation into the (E)-allylsilane and the 6-membered disiladioxane. Subsequent treatment of the mixture with n-butyllithium results in Peterson-type elimination of the latter to give (E)-allylsilane. The chirality of the starting allylic alcohol was stereospecifically transferred into the produced (E)-allylsilane. Notable is that (E)- and (Z)-allylic alcohols with the same chirality provided (E)-allylsilanes with the opposite absolute configuration.