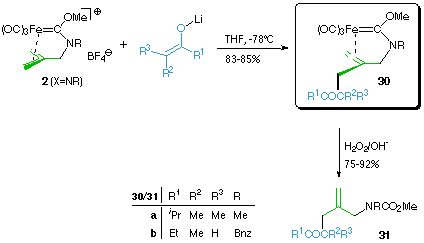

2 (X=NR) readily reacts with carbon nucleophiles like lithium enolates or heteroatom nucleophiles like phosphanes to give isolable alkene-carbene complexes 30 as products of a nucleophilic attack on a terminal allylic atom. They can be oxidatively demetalated to give the corresponding allylic carbamates 31 in good to excellent yields. So in four steps both hydroxy groups of the starting isobutene diol can be substituted, the first one by a heteronucleophilic amine, the second one by a C-nucleophilic lithium enolate to give carbamates with the possibility of introducing various residues at virtually each position of the beta-methylene-eta-oxo-
substituted carbamate skeleton[3].
Experimental data
1. Synthesis of alkene–carbene complexes 30 (general procedure):
2. Synthesis of allyl carbamates 31:
First solutions of the required lithium enolates in THF were prepared by treating a solution of isopropyl cyclohexylamine (164 ml; 1.1 mmol) in THF (5 ml) with a 2.5 M solution of nBuLi in hexane (0.44 ml; 1.1 mmol) at 0°C. The mixture was stirred for 30 min, then chilled to -78°C and subsequently treated with the carbonyl compound (1.1 mmol). After stirring at -78°C for 60 min, the resulting enolate solution was transferred via a cannula into a slurry of the respective carbene complex 2b (1.0 mmol) in THF (5 ml) kept at -78°C as well. The entire mixture was stirred for a further 2 h at -78°C, then allowed to warm up to room temperature and all volatile components were finally removed in vacuo. The resulting residue was repeatedly extracted with diethyl ether (3 x 10 ml), the combined extracts were concentrated on an oil pump and then purified by CC (silica gel; diethyl ether / petrol ether 1:2).
Tricarbonyl[(4-5-eta2)-2-aza-1-methoxy-2-methyl-4-(3'-oxo-2',2',4'-trimethyl) pentyl-pent-4-en-1-ylidene]iron(0) (30a):
IR (KBr): 3040, 2960, 2920, 2860, 2000, 1940, 1910, 1690, 1545, 1460, 1230 cm-1. - 1H NMR (C6D6, 400 MHz): 0.95 [d, 3J (CH3/4'-H) = 6.60 Hz, 3 H, 5'-H], 0.96 (s, 3 H, 2'-CH3), 0.99 [d, 3J (CH3/4'-H) = 6.60 Hz, 3 H, 4'-CH3], 1.12 (s, 3 H, 2'-CH3), 1.74 (s, 1 H, 5-H), 1.83 [d, 2J (1'-H/1'-H') = 14.30 Hz, 1 H, 1'-H], 1.96 (s, 3 H, NCH3), 2.19 [s, 1 H, 5-H'], 2.75 [qq, 3J (4'-H/CH3) = 6.60 Hz, 1 H, 4'-H], 3.15 [d,
2J (1'-H/1'-H') = 14.30 Hz, 1 H, 1'-H'], 3.21 [d, 2J (3-H/3-H') = 12.65 Hz, 1 H, 3-H], 3.41 [d, 2J (3-H/3-H') = 12.65 Hz, 1 H, 3-H'], 3.71 (s, 3H, OCH3).-13C NMR (C6D6, 100.5 MHz): d = 20.62 and 20.66 (C-5', 4'-C), 23.74 and 26.78 (2'-C), 32.63 (NCH3), 34.49 (C-4'), 42.44 (C-1'), 50.28 (C-2'), 52.63 (C-5), 62.39 (OCH3), 62.85 (C-3), 63.31 (C-4), 216.84 [Fe(CO)], 218.26 (C-3'), 241.14 (C-1). - MS (70 eV); m/z (%): 351 (8) [M+ - CO], 323 (22) [M+ - 2 CO], 295 (90) [M+ - 3 CO], 183 (100), 95 (68). - C17H25FeNO5 (379.2): calcd. C 53.84, H 6.65, N 3.39; found C 54.01, H 6.73, N 3.55.
Solutions of the carbene complexes 30 (1.1 mmol) in methanol (20 ml) were chilled to 0°C and treated with H2O2 (6 ml of a 30% aqueous solution; 6.0 mmol). Then solid sodium hydroxide (240 mg; 6 mmol) was added within 2 h and stirring was continued for another 2 h. Finally, the mixtures were extracted thrice with diethyl ether (100 ml) and the combined extracts were washed with saturated aqueous NH4Cl solution and dried over MgSO4. After removal of the solvent on a rotary evaporator, the crude products were purified by CC (diethyl ether / petrol ether 1:2).
O-Methyl,N-methyl,N-(2-methylene-4,4,6-trimethyl-5-oxo)heptyl carbamate (31a):
IR (neat): 2960, 2930, 1705, 1470, 1390, 1260, 1090 cm-1. - 1H NMR (CDCl3, 400 MHz): d = 1.05 [d, 3J (6-H/CH3) = 6.84 Hz, 6 H,
6-CH3, 7-H], 1.20 (s, 6 H, 4-CH3), 2.23 (s, 2 H, 3-H), 2.82 and 2.87 (s, 3 H, NCH3), 3.11 [sep, 3J (6-H/CH3) = 6.84 Hz, 1 H, 6-H], 3.68 (s, 2 H, 1-H), 3.71 (s, 3 H, OCH3), 4.81 and 4.89 (m, 2 H, =CH2).-13C NMR (CDCl3, 100.5 MHz): d = 20.19 (C-7, 6-CH3), 24.48 (4-CH3), 33.65 (NCH3), 34.37 (C-6), 41.40/41.71 (C-3), 48.07 (C-4), 52.67 (OCH3), 54.72/55.19 (C-1), 113.64/114.24 (=CH2), 141.08 (C-2), 157.14 (NCO), 219.64/219.77 (C-5). – MS (70 eV); m/z (%): 255 (10) [M+], 184 (18) [M+ - iPrCO], 142 (100), 110 (20), 95 (98), 43 (85) [iPr+]. - C14H25NO3 (255.3): calcd. C 65.85, H 9.87, N 5.48; found C 65.83, H 9.90, N 5.40.