| [Related articles/posters: 005 023 032 ] |

C-C Bond Formation with Chelated Alkene-Carbene and Allyl-Carbene Complexes
Novel Pathways to Oligo-enes, Oligo-en-ones, Oligo-en-ynes, Allylcarbamates and -alcohols, delta-Lactones and Cyclopentenones
Introduction
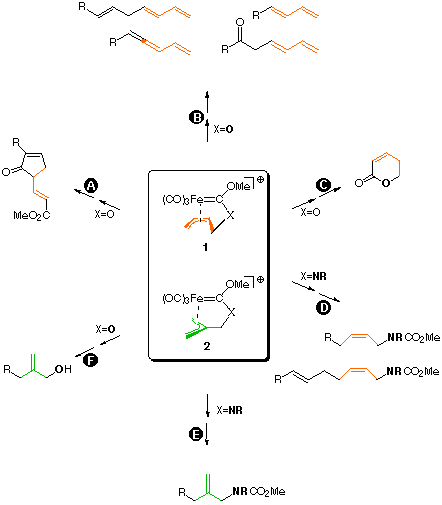
Chelated alkene-carbene complexes are useful model systems for studying transition-metal-mediated olefin reactions like metathesis etc. as their reactivity chiefly depends on the tether length. Their synthetical value is due to their inherent chirality and to the chance for well-defined, concerted multi-step processes including carbene, alkene and tether moieties. Chelated allyl carbene complexes like 1 and 2 are less well investigated, although offering a more flexible reactivity pattern, especially towards carbon and heteroatom nucleophiles, which may attack at different sites depending on the particular set of substituents and the nucleophile. Reactions with enolates, cuprates, acetylides, or phosphanes at distinct sites of the metallacyclic perimeter of 1 provide an easy access to unsaturated organic products like highly functionalized cyclopentenones (path A), oligo-enes, oligo-en-ones, oligo-en-ynes (path B), delta-lactones (path C) and carbamates (path D). Reactions of nucleophiles with 2 lead to allylcarbamates (path D) or -alcohols (path E). Applications to the synthesis of natural products like pheromones and terpenes are given. For reviews see [1]