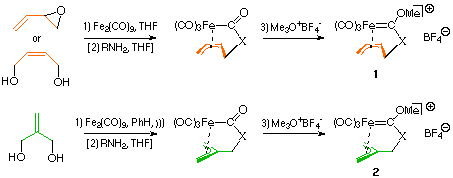
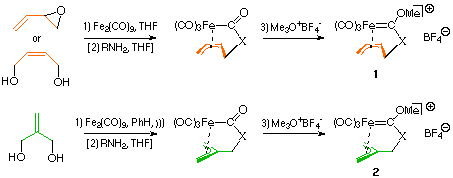
| The starting complexes 1 and 2 are easily accesible in two (for X=O) or three (for X=NR) steps, respectively, from iron carbonyls and vinyl oxiranes or butendiols [2],[3]. The ferralactones [4] formed in the first step can be alkylated with Meerwein salts either directly or after aminolysis with primary amines to give the corresponding carbene complexes with X=O or X=NR. | X-ray structure of 1: |