| [Related articles/posters: 028 039 091 ] |
A new one-pot synthesis of 2-aryl-1,3-oxazin-6-ones by reaction of 4-acyloxyazetidin-2-ones with aroyl chlorides, under mild conditions, is described.
![]() Introduction
Introduction
1,3-Oxazin-6-ones (3-aza-a-pyrones) 1 are six membered heterocyclic compounds which have been appreciated as important substrates in heterocyclic transformations, [1] specially in [4 + 2] cycloaddition reactions of its 2-azadiene moiety with a variety of dienophiles leading to pyridines, after extrusion of CO2. [1,2]
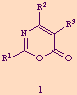
The parent compound 1 (R1 = R2 = R3 = H) was prepared for the first time in 1975, [3] and a detailed survey of the general synthetic methods leading to the preparation of substituted derivatives, covering the literature up to 1986, is available.[1]
By far the most general way to synthesize 1,3-oxazin-6-ones 1 is the cyclization of 3-acylaminoacrylic acids and esters 2, usually by thermal methodologies, through the pressumed intermediacy of acyliminoketenes 3 which experienced electrocyclization to heterocycles 1.
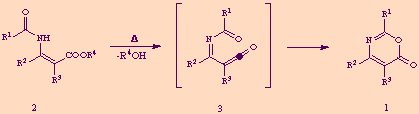
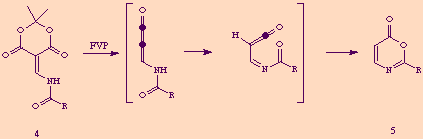
Herein we report a new method for the preparation of compounds 5 under mild conditions, avoiding FVP techniques, starting from commercially available ß-lactams.
![]() Results and discussion
Results and discussion
During the course of an investigation on intramolecular aza-Wittig reactions of the ß-lactam carbonyl group, [5] we attempted to acylate the nitrogen atom of 4-acetoxy-2-azetidinone 6 with 2-azidobenzoyl chloride under basic conditions. In that reaction we obtained, together with the desired N-acyl-ß-lactam 7, a minor amount of a previously unknown compound to which we assigned the structure 2-(2-azidophenyl)-1,3-oxazin-6-one 8 on the basis of its analytical and spectral data.
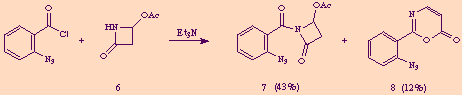
Compound 8 was also obtained as a byproduct in the acylation of 4-benzoyloxy-2-azetidinone 6' with the same aroyl chloride.
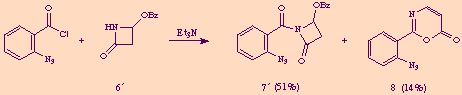
At first sight, compound 8 seemed to derive from N-acyl-2-azetidinones 7 and 7' through elimination of acetic and benzoic acid respectively, accompanied by a molecular reorganization. We could in fact easily demonstrate this assumption just exposing pure 7 or 7' to the action of one equivalent amount of triethylamine, thus obtaining 8 in good yields.
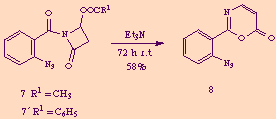
As expected, when the acylation of 6 or 6' with 2-azidobenzoyl chloride was carried out in the presence of more than two equivalents of base (usually Et3N) in dichloromethane solution, and the reaction time was extended to 16-20 h, the only reaction product isolated was the 1,3-oxazin-6-one 8.
This one-pot preparation of 2-substituted 1,3-oxazin-6-ones from commercially available 4-acetoxy or 4-benzoyloxy-2-azetidinones and acyl chlorides has been found to be of wide applicability, as reflected in the following scheme and table.
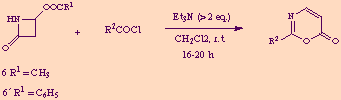
| Starting from | R2 | Yield (%)a |
| 6 | 2-N3-C6H4 | 50 |
| 6´ | 2-N3-C6H4 | 58 |
| 6 | 4-NO2-C6H4 | 63 |
| 6´ | 4-NO2-C6H4 | 70 |
| 6´ | 4-CH3-C6H4 | 65 |
| 6´ | C6H5 | 76 |
| 6 | (E)-C6H5-CH=CHb | 45 |
| 6´ | (E)-C6H5-CH=CHb | 50 |
| 6´ | (E)-4-CH3O-C6H4-CH=CHb | 49 |
| 6 | 2-Furyl | 67 |
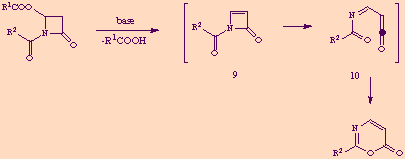
We believe that the mechanism of the conversion 7 to 8 involves initial ß-elimination of carboxylic acid from the azetidinone ring giving rise to the reactive, strained azetinone 9. Ring opening of this intermediate would lead to the acyliminoketene 10 which finally electrocyclized to the 1,3-oxazin-6-one ring.
We are currently investigating on the applicability of the present method to the synthesis of 2-alkyl-1,3-oxazin-6-ones, as well on attempts to trap intramolecularly the proposed intermediate acyliminoketene 10 by attaching adequate functionalities to the R2 group of the starting acyl chloride.
![]() Acknowledgements
Acknowledgements
Thanks are given to Dirección General de Investigación Científica y Técnica for financial support (project number PB95-1019).