| [Related articles/posters: 028 111 053 ] |
Although considerable efforts1,2 have been devoted to the syntheses of psilocin1 (1), psilocybin1 (2), and their analogs, there still remains to establish structure-activity relationships. With a hope to find a lead compound among psilocin analogs possesing suitable pharmacological effects for psychotic diseases such as depression, schizophrenia, Alzheimer's disease, and so on, we have developed a novel five-step preparative method of 1 from indole-3-carbaldehyde (3).
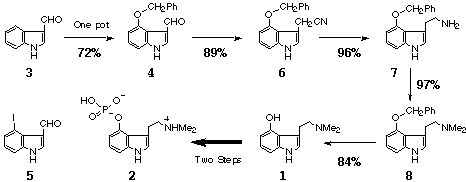
We have attempted to improve the yield of 4 as well as the reproducibility in order to utilize the one pot procedure as the first step in the preparative method of 1. Among the three reactions involved, we thoroughly reexamined the third reaction of 4-iodoindole-3-carbaldehyde (5) with metal benzyloxide (20-26 mol eq) in the presence of CuI (3 mol eq) in DMF at 1200C.3 Consequently, we found that not only the preparation method of metal benzyloxide but also the metal itself is an important factor for controlling the overall yield. Thus, treatment of 5 with sodium benzyloxide (NaOCH2Ph), prepared from sodium and benzyl alcohol (PhCH2OH), afforded 4 in 24% yield. When NaH was employed to produce NaOCH2Ph, the yield doubled to 48%. In contrast, when potassium was used instead of sodium, further improvement in the yield was observed (54%). Finally, KH was found to be the reagent of choice and thereby the yield was raised up to 70%. Among the tested solvents, DMF was found to give the best yield of 4. Replacement of DMF by either HMPA or PhCH2OH dropped the yield to 48 or 30%, respectively. We have further reconfirmed that the suitable amount of KOCH2Ph is 25 mol eq to the starting material 3.
The other important factor to keep reproducibility in the one pot procedure was the relative ratio of iodination reagents (I2 and CuI) to KOCH2Ph. Fixing the amount of KOCH2Ph to about 25 mol eq, the ratio was examined culminating in finding that 3.0 mol eq to 3 was the best reaction conditions, where the desired 4 was produced in 72% overall yield from 3 with an excellent reproducibility.
As the second step in the preparative method of 1, 4 was transformed into 4-benzyloxyindole-3-acetonitrile (6) in 89% yield utilizing our reaction,4 thus by treating 4 with NaBH4 in the presence of NaCN in MeOH and formamide. Reduction of 6 with LiAlH4 in Et2O is the third step giving the known 4-benzyloxytryptamine1a (7) in 96% yield. Interestingly, when THF was used as a solvent instead of Et2O, the yield of 7 decreased dramatically to the range of 25 to 46%. As the fourth step, dimethylation of 7 was carried out with formaldehyde and sodium cyanoborohydride5 in AcOH to produce 81c in 97% yield. The final step of debenzylation of 8 was the catalytic hydrogenation using 10% Pd/C producing psilocin1d (1) in 84% yield. Since 1 was converted to psilocybin (2) in two steps by Hofman and co-workers,1c the present method constitutes a seven-step synthesis of 2 as well.
In conclusion, we could establish a novel five-step preparative method of 1 in 50% overall yield and 50% originality rate.6 Utilizing 1 and its intermediates (4, 6-8), the syntheses of psilocin analogs and particularly the compounds having substituents in the benzene part are in progress.
References and Notes