| [Related articles/posters: 078 074 023 ] |
Introduction
3-Oxobenzo[b]furans, thiophenes and selenophenes have shown great interest over the years for their synthetic utility. We present here some synthetic transformations which enable the oxo derivatives to become a useful starting material for building 6-methylbenzohetero[2,3-c]quinolines and 5-methylbenzohetero[2,3-c]isoquinolines via cross-coupling reactions.
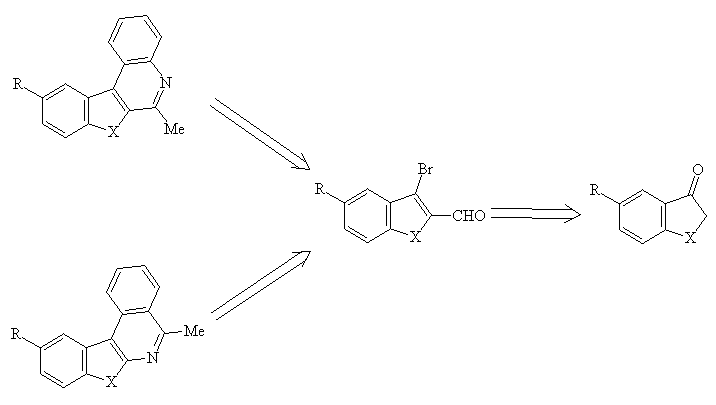
Synthesis of 2-carboxaldehyde-3-phenylbenzo[b]furans, thiophene and selenophene and 2-acetyl-3-phenylbenzo[b]furans, thiophene and selenophene
The synthesis of 2-carboxaldehyde (or 2-acetyl) 3-phenylbenzo[b]furans, thiophene, selenophene has been performed starting from 3-bromo-2-carboxaldehydebenzo[b]furans, thiophene and selenophene 2(a-e) or from the 2-acetyl-3-trifluoromethylsulfonylbenzo[b]furans 3.
é From the 3-bromo-2-carboxaldehydebenzo[b]furans, thiophene and selenophene
The synthesis of 3-bromo-2-carboxaldehydebenzo[b]furans, thiophene and selenophene 2(a-e) has been achieved using the well-known Vilsmeier-Haack-Arnold reaction(1) (Scheme 1).
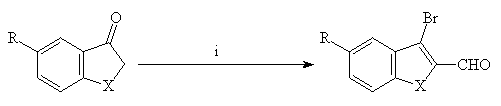
1(a-e) 2(a-e)
X = O, S, Se
R = H, OMe, SMe
Scheme 1 : Reagents and conditions : i : POBr3, DMF, CHCl3, 70°C, 6 h
Yields : 72-97%
The 3-bromo-2-carboxaldehydebenzo[b]furans, thiophene and selenophene were reacted either under Stille conditions(2) (with an organostannane) or under Suzuki conditions(3) (with an organoboronic acid).
We first studied the cross-coupling of phenyltributylstannane with the bromo derivatives 2(a-e) under Stille conditions(2). Thus, treatment of compounds 2(a-e) with phenyltributylstannane in the presence of catalytic palladium (0) afforded the corresponding product 3(a-e) respectively, together with some starting material. The results are given in table I.
Table I : Coupling of phenyltributylstannane with the 3-bromo-2-carboxaldehyde derivatives 2(a-e)
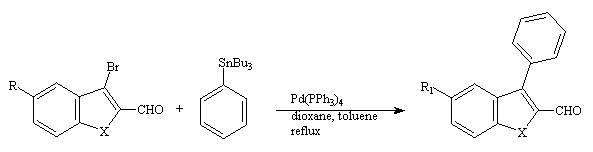
2(a-e) 3(a-e)
|
Compound |
X |
R |
% isolated yield of product 3 |
|
3a |
O |
H |
76 |
|
3b |
O |
OMe |
70 |
|
3c |
O |
SMe |
84 |
|
3d |
S |
H |
72 |
|
3e |
Se |
H |
92 |
As it can be seen, the reaction of phenyltributylstannane with the 3-bromo-2-carboxaldehyde 2(a-e) gave good yields of the desired 2-carboxldehyde-3-phenyl compounds 3(a-e).
Next, we investigated the coupling of phenylboronic acid with the bromo derivatives 2(a-e) under the Suzuki reaction conditions(3). Compounds 2(a-e) were heated with phenylboronic acid in the presence of 3% Pd(PPh3)4 in dimethoxyethane. Aqueous potassium carbonate (2 eq.) was added to catalyse the reaction. The results of this study are shown in table II.
Table II : Coupling of phenylboronic acid with the 3-bromo-2-carboxaldehyde derivatives 2(a-e)
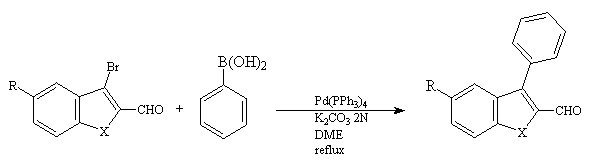
2(a-e) 3(a-e)
|
Compound |
X |
R |
% isolated of product 3 |
|
3a |
O |
H |
87 |
|
3b |
O |
OMe |
65 |
|
3c |
O |
SMe |
91 |
|
3d |
S |
H |
86 |
|
3e |
Se |
H |
81 |
Again one can see that the isolated yields of coupling are good with the 3-bromo-2-carboxaldehyde derivatives 2(a-e).
é From the 2-acetyl-3-trifluoromethanesulfonylbenzo[b]furans 5
The reaction of the enols 4(a-c) with trifluoromethanesulfonic anhydride in dichloromethane under usual conditions(4) led to the 2-acetyl-3-trifluoromethylsulfonyl derivatives 5(a-c) (Scheme 2).
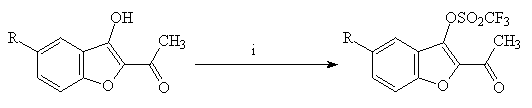
4(a-c) 5(a-c)
Scheme 2 : Reagents and conditions : i : O(SO2CF3)2, CH2Cl2, pyridine, r.t., 12h (61-94%)
Under standard Stille conditions(5) (phenyltributylstannane, Pd(PPh3)4, LiCl) or Suzuki conditions(6) (phenylboronic acid, Pd(PPh3)4, Na2CO3), reaction of the triflates 5(a-c) gave mostly 2-acetyl-3-phenyl benzo[b]furans 6(a-c) (Scheme 3).
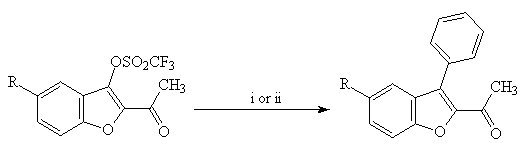
5(a-c) 6(a-c) yields = 70-91%
Scheme 3 : Reagents and conditions : i : PhSnBu3, Pd(PPh3)4, LiCl, THF, reflux; ii : PhB(OH)2, Pd(PPh3)4, Na2CO3, CuI, toluene , reflux
Access to 6-methylbenzohetero[2,3-c]quinolines
é Preparation of 2-acetyl-3-phenylbenzo[b]thiophene and selenophene 6(d-e) :
Reaction of the carboxaldehydes 3(d-e) with Grignard reagent (CH3MgI) in ether followed by the oxydation of the corresponding alcohols with PCC in dichloromethane afforded the 2-acetyl-3-phenylbenzo[b] thiophene and selenophene 6(d-e) in good yields (Scheme 4).
Scheme 4 : Synthesis of 2-acetyl-3-phenylbenzo[b]thiophene 6d and 2-acetyl-3-phenylbenzo[b]selenophene 6e
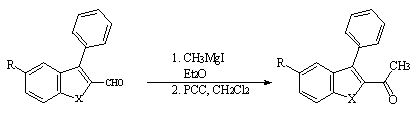
3(d-e) 6(d-e)
The 6-methylbenzohetero[2,3-c]quinolines have been synthesized starting from the 2-acetyl-3-phenylbenzo[b]furans, thiophene and selenophene 6(a-e).
First, we prepared the corresponding oximes 7 in high yields by treatment of ketones 6(a-e) with hydroxylamine hydrochloride in the presence of sodium acetate in ethanol (Scheme 5).
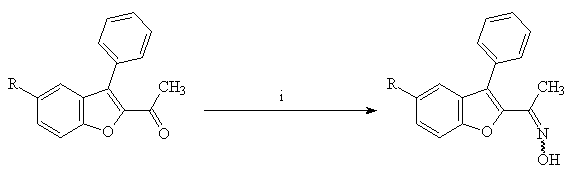
Scheme 5 : Reagents and conditions : i : NH2OH.HCl, AcONa, EtOH (yields > 90%).
To explore the scope of the substitution reaction on the nitrogen atom of oximes 7, the cyclisation was examinated by using the 2,4-dinitrophenyl derivatives 8. Treatment of 8 with sodium hydride in refluxing 1,4-dioxane(7) afforded the 6-methylbenzohetero[2,3-c]quinolines in good yields (Scheme 6).
Scheme 6 : Synthesis of 6-methylbenzohetero[2,3-c]quinoline 9(a-e)
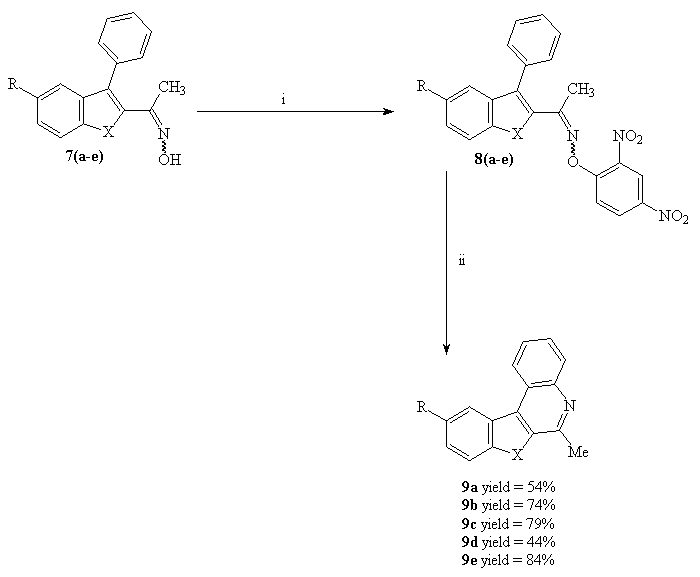
Reagents and conditions : i : 2,4-dinitro-chlorobenzene, KOH, THF (yields = 77-89%); ii : NaH, 1,4-dioxane, reflux (yields = 44-84%).
Access to 5-methylbenzohetero[2,3-c]isoquinolines
The 5-methylbenzohetero[2,3-c]isoquinolines were prepared from the 2-acetyl-3-phenylbenzo[b]furans, thiophene and selenophene 6(a-e) by an oximation-Beckmann transposition-Bischler-Napieralski cyclisation sequence (Scheme 7).
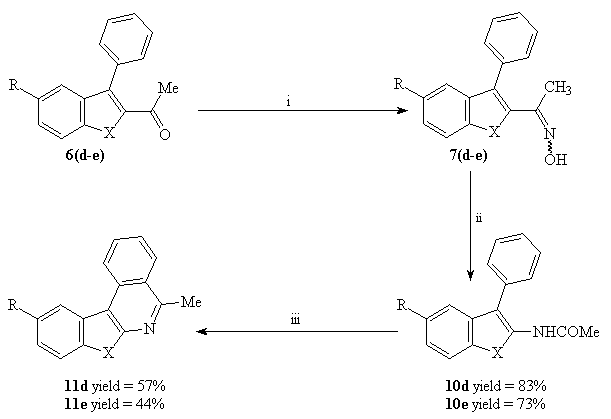
Scheme 7 : Reagents and conditions : i : NH2OH.HCl, AcONa, EtOH ( yields > 90% ); ii : PPA, toluene, reflux ( yields = 71-83%); iii : POCl3, CH2Cl2, reflux.
Conclusion
The Stille and Suzuki cross-coupling reactions applied to 3-bromo-2-carboxaldehydebenzo[b]furans, thiophene and selenophene and to 2-acetyl-3-trifluoromethanesulfonylbenzo[b]furans have led to benzo[b]furans , thiophene and selenophene substituted by a phenyl group in the position 3 with good yields.
Moreover, the derivatives having a carboxaldehyde or an acetyl group in position 2 lead to tetracyclic compounds with potential pharmacological interest.
References
1. A. Ricci; D. Balucani; C. Rossi; A. Croisy; Boll.Sci.Fac.Chim.Ind.Bologna, 1969, 27, 279
2. a) J.K. Stille; Angew.Chem.Int.Ed.Engl., 1986, 25, 508
b) S. Gronowitz; A. Messmer; G. Timari; J.Heterocyclic Chem, 1992, 29, 1049
c) T.N. Mitchell; Synthesis, 1992, 803
d) G. Martorell; A. Garcia-Raso; J.M. Saa; Tetrahedron Lett.,1990, 31, 2357
3. a) N. Miyaura; T.Yanagi; A. Suzuki; Synth.Commun., 1981, 11, 513
b) S. Gronowitz; V. Bobosik; K. Lawitz, Chem.Scr., 1984, 24, 5
c) A. Suzuki; Pure Appl. Chem., 1994, 66, 213
d) S. Gronowitz; K. Lawitz; Chem.Scr., 1984, 23, 120
e) T. Watanabe; N. Miyaura; A. Suzuki; Synlett, 1992, 207
4. P.J. Stang; M. Hanack; L.R. Subramanian; Synthesis, 1982, 85
5. a) A.M. Echavarren; J.K. Stille; J.Am.Chem.Soc., 1987, 109, 5478
b) J.M. Saa; G. Martorell; J.Org.Chem, 1993, 58, 1963
c) W.J. Scott; G.T. Crisp; J.K. Stille; J.Am.Chem.Soc., 1984, 106, 4630
d) J.A.Robl; Tetrahedron Lett., 1990, 31, 3421
6. a) A. Huth; I. Beetz; I. Schumann; Tetrahedron, 1989, 45, 6679
b) J.M. Fu; V. Snieckus; Tetrahedron Lett., 1990, 31, 1665
c) T. Oh-e; N. Miyaura; A. Suzuki; J.Org.Chem., 1993, 58, 2201
7. K. Uchiyama; Y. Hayashi; K. Narasaka; Synlett, 1997, 445