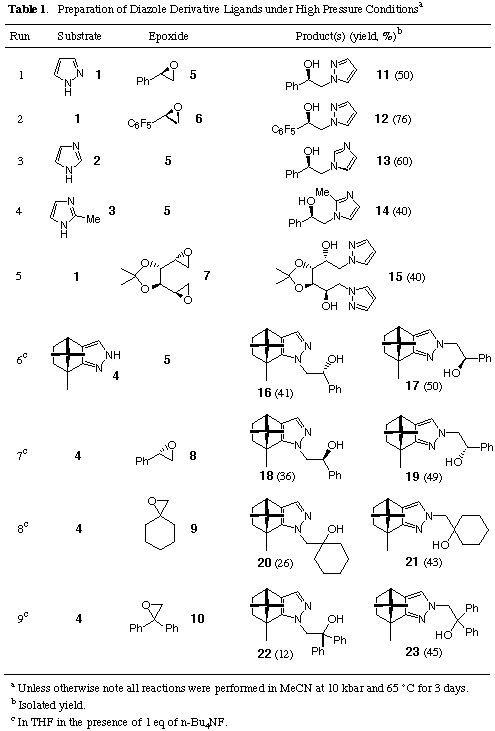Novel chiral pyrazole ligands for enantioselective addition of diethylzinc to benzaldehyde
Hiyoshizo Kotsuki,* Masahiro Wakao and Hiroyuki Hayakawa
Department of Chemistry, Faculty of Science, Kochi University, Akebono-cho,
Kochi 780, Japan
Introduction
The molecular design of enantiomerically pure chiral ligands for use in
catalytic asymmetric reactions is one of the most challenging issues in modern
organic chemistry.1 In our extensive efforts in this area, we
recently disclosed an efficient procedure to obtain chiral
pyridine,2 diamine,3 and diazole derivatives.4
Herein, we describe a general procedure for preparing a variety of chiral
diazole ligands having an alcoholic side chain and also demonstrate their
utility in catalytic asymmetric alkylation of benzaldehyde with diethylzinc.
Results and discussion
Our new procedure for the synthesis of chiral diazole derivative ligands
relies on high pressure promoted N-alkylation of pyrazoles or imidazoles
with optically active epoxides as depicted in Scheme 1.5
The results are summarized in Table 1. For example, the reaction of
(+)-camphorpyrazole 46 with (R)-(+)-styrene oxide
5 at 10 kbar in the presence of 1.0 equiv. of Bu4NF gave
16, mp 150.5-152 oC (from hexane-Et2O); [a]25D +65.7
(c 0.35, EtOH), and 17, [a]25D +47.8 (c
1.38, EtOH), in 41 and 50% yield, respectively (Run 6). Similarly, other
derivatives were also prepared without difficulty.
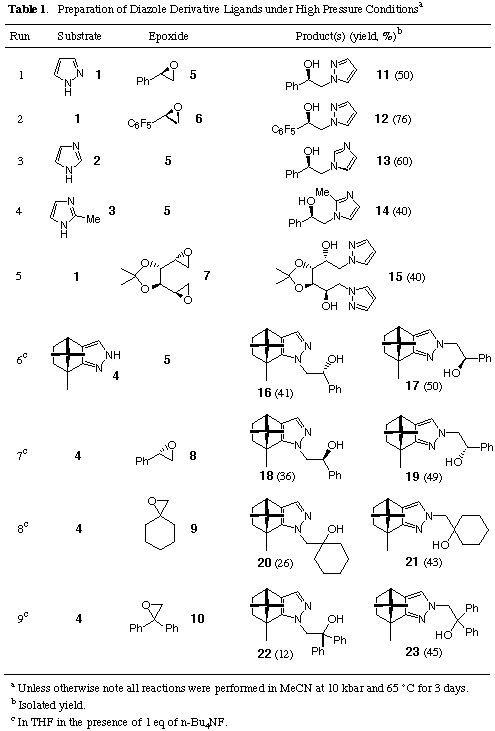
With these ligands in hand, the catalytic activities of 11-23 for
the enantioselective addition of diethylzinc to benzaldehyde was then examined
(Scheme 2 and Table 2).7

As expected, a relatively high enantiomeric excess (83% ee) was obtained when
the pyrazolyl ligand 11 was employed as a chiral catalyst (Entry 1),
whereas the corresponding imidazole homologues were all fruitless (Entries 6
and 7). These results can be understood by considering the favourable
coordination structure of 11 to form a six-membered organozinc
intermediate. After numerous attempts to improve the enantioselectivity, we
finally found that the sterically congested ligand 16 gave the best
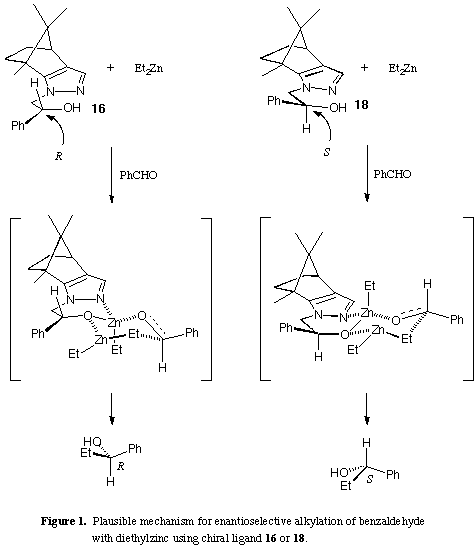
result: 93% ee was achieved in favour of an R-enantiomer (Entry 10).
Interestingly, simply by using the diastereomer 18, in which the
stereogenic carbon centre bearing a hydroxy function has an
S-configuration, the completely reversed enantioselectivity was effected
in 93% ee (Entry 14). Interestingly, the catalytic activity of these ligands
survived even at low concentration (0.05 equiv., Entries 12 and 16).
From these results, we proposed the transition state structures for the present
reaction as outlined in Fig. 1 wherein the steric environment around the
hydroxy group plays an important role to provide the desired
enantioselectivity.
Conclusion
We were successful in developing a convenient method to synthesize a variety of
diazole chiral ligands using high pressure-promoted epoxide opening reactions,
and in clarifying the novel catalytic activity of these ligands in
enantioselective addition of diethylzinc to benzaldehyde. The best results for
the chiral catalysts 16 and 18 were 93% ee in either case,
wherein reversed asymmetric induction was achieved. In view of the ready
accessibility of these chiral ligands, the present method offers potential
utility for the design of other types of chiral ligands.
Acknowledgments
The present work was partially supported by Scientific
Research Grants (Nos. 05453063 and 07304047) from the Ministry of Education,
Science and Culture of Japan. We also thank the Asahi Glass Foundation and the
Naito Foundation for financial support of this work.
- Togni, A. Tetrahedron: Asymmetry, 1991, 2, 683;
ed. Ojima, I., Catalytic Asymmetric Synthesis; VCH: Weinheim, 1993;
Noyori, R. Asymmetric Catalysis in Organic Synthesis; Wiley: New York,
1994; Togni, A.; Venanzi, L. M. Angew. Chem., Int. Ed. Engl.
, 1994, 33, 497.
-
Kotsuki, H.; Nakagawa, Y.; Moriya, N.; Tateishi, H.; Ochi, M.; Suzuki, T.;
Isobe, K. Tetrahedron: Asymmetry, 1995, 6, 1165.
-
Kotsuki, H.; Kuzume, H.; Gohda, T.; Fukuhara, M.; Ochi, M.; Oishi, T.;
Hirama, M.; Shiro, M. Tetrahedron: Asymmetry, 1995, 6,
2227.
-
Kotsuki, H.; Hayakawa, H.; Wakao, M.; Shimanouchi, T.; Ochi, M.
Tetrahedron: Asymmetry, 1995, 6, 2665.
-
Review: Kotsuki, H. Kagaku to Kogyo (Osaka), 1994, 68,
265; Kotsuki, H.; Hayashida, K.; Shimanouchi, T.; Nishizawa, H., J. Org.
Chem., 1996, 61, 984.
-
Brunner, H.; Scheck, T. Chem. Ber., 1992, 125, 701;
Christenson, D. L.; Tokar, C. J.; Tolman, W. B. Organometallics
1995, 14, 2148. For the use of chiral pyrazole derivatives as a
chiral auxiliary, see: Kashima, C.; Fukuchi, I.; Takahashi, K.; Hosomi, A.
Tetrahedron Lett., 1993, 34, 8305.
-
Noyori, R.; Kitamura, M. Angew. Chem., Int. Ed. Engl., 1991, 30, 49; Soai, K. Chem. Rev., 1992, 92, 833; P. Knochel and R. D.
Singer, Chem. Rev., 1993, 93, 2117.