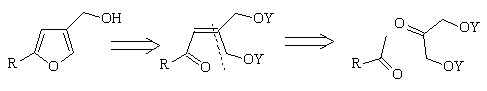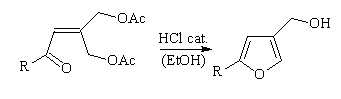A new approach to 2-substituted-4-furanmethanol compounds
División de Estudios de Posgrado, Facultad de Química, UNAM,
Circuito Exterior, Ciudad Universitaria, Coyoacán 04510, México
D.F., México
Abstract
A two step synthesis of the title compounds from readily available
starting materials is reported. The method involves a
Hörner-Wadsworth-Emmons reaction between b-ketophosphonates anions
and 1,3-diacetoxy-2-propanone, followed by mild acid treatment of the
g,g'-diacetoxyenones thus obtained.
Background: the total synthesis of racemic bilobanone
In 1980 a total synthesis of the sesquiterpene bilobanone 5 from
2-isobutyl-4-furanmethanol 4a was reported (Escalona, H.; Maldonado, L.A., Synth. Commun., 1980,10, 857) from our laboratory. The
synthesis of 4a involved a new method for the preparation of
2-substituted-4-functionalized furans in which the heterocyclic ring was
formed by acid hydrolysis of nitroketal diol 1, followed by base
treatment of the nitroketone diol 2 intermediate.

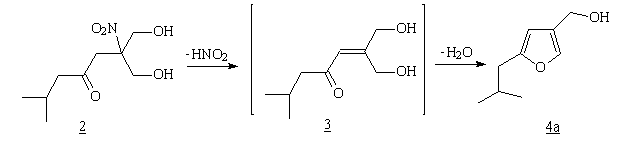
Scheme 1
Proposed reaction mechanism for the conversion
2 TO 4a
Although the reaction mechanism for the conversion 2 -> 4a is
unknown. In our 1980 paper a completely different reaction mechanism was proposed for
this conversion. However, with the recent isolation of 2 as intermediate
in this transformation, we have changed our original proposal., from our point of view it was reasonable to assume that HNO2
elimination in nitroketone diol 2 would afford an undetected
intermediate g,g'-dihydroxyenone 3 which cyclizes very rapidly
to the furan ring.
Scheme 2
Working hypothesis: g,g'-dihydroxyenones as precursors of
2-substituted 4-furanmethanol compounds
Accordingly with the proposed reaction mechanism, we reasoned
that if undetected intermediate g,g'-dihydroxyenone 3 (or a
conveniently protected derivative) could be prepared by a direct method, a
simple synthesis of furans such as 4 would be at hand.
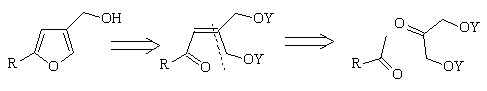
Scheme 3
The experimental demonstration of this working hypothesis
strongly suggests (although does not prove) that our proposed reaction
mechanism is correct.
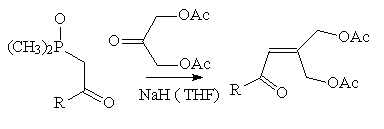
Synthesis of g,g'-diacetoxyenones through the
HÖrner-Wadsworth-Emmons reaction
For its convergency, simplicity and generality, the method of enone formation
we choose, was the Hörner-Wadsworth-Emmons reaction between
b-ketophosphonate anions and 1,3-diacetoxypropan-2-one (Bentley, P.H.; McCrae, W., J. Org. Chem., 1970, 35, 2082.) as the carbonyl
acceptor.
Scheme 4
R= (CH3)2CHCH2 77%
R= C6H5 65%
R=2,5-(OCH3)2C6H3, 80%
Although we have found that 1,3-diacetoxypropan-2-one is a highly convenient
(easily obtained, stable crystalline solid) substrate for the
Hörner-Wadsworth-Emmons reaction many other OH-protected
1,3-dihydroxyacetone derivatives are also conceivable candidates.
Synthesis of 2-substituted-4-furanmethanol compounds
Our first attempts to convert the g,g'-bisacetoxyenones to the
title furans under basic conditions failed. However, in acidic medium
(catalytic aqueous HCL in EtOH, 65 oC, 4-5 h) they were obtained in good to
moderate yields (52-85%). In some experiments, small amounts of the corresponding acetates have been isolated.
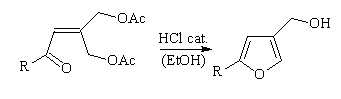
Scheme 5
4a, R= (CH3)2CHCH2 76%
4b, R= C6H5 85%
4c, R=2,5-(OCH3)2C6H3, 52%
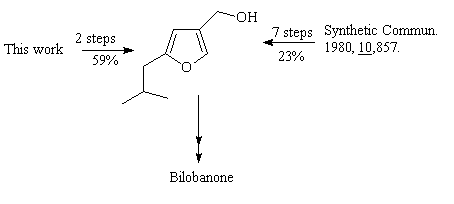
Conclusion (I)
2-Isobutyl-4-furanmethanol 4a, an intermediate in our bilobanone
synthesis, has now been obtained in two simple steps and in 59% overall yield.
This is a considerable improvement over our original procedure (~ 23% overall
yield through seven steps).
Scheme 6
Conclusion (II)
2-(2,5-dimethoxyphenyl)-4-furanmethanol 4c is an easily available,
potential intermediate for shikonofuran and echinofuran syntheses. (Inovye, H.; Matsumara, H.; Kawasaki, M.;Inove, K.; Tsukada, M.; Tabata, M.
Phytochemistry, 1981,20,1701;
Yoshizaki, F.; Hisamichi, S.; Kondo, Y.; Sato, Y.; Nozoe, S., Chem. Pharm.
Bull., 1982,30,4407.

Scheme 6