| [Molecules: 6] [Related articles/posters: 005 074 036 117 056 ] |
We now report the synthesis of some compounds including combination of pyrazole or pyrimidine ring with imidazole ring and a saccharide component in the molecule.
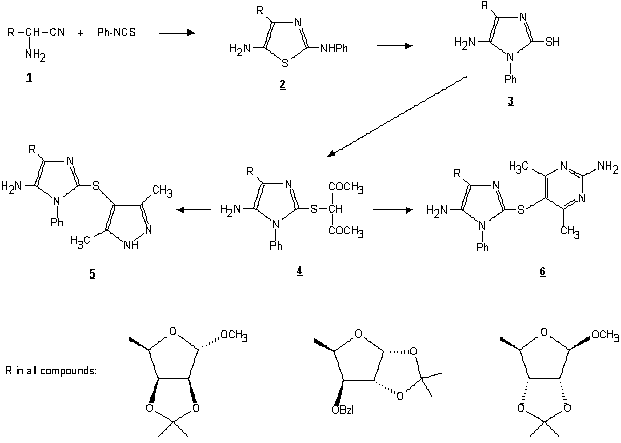
Starting from the known methyl 2,3-O-isopropylidene-a-D-lyxo-pentodialdo-1,4-furanoside [1], the corresponding a-aminonitrile 1 was prepared as a mixture of diastereomers (considering that the C-5 asymmetric centre is destroyed in the next reaction step, separation of diastereomers was unnecessary) by the Strecker reaction [2]. Compound 1 reacted with phenyl isothiocyanate to give thiazole 2 which subsequently rearranged to 2-mercaptoimidazole 3upon treatment with base. Following S-alkylation of 3 with 3-chloro-2,4-pentanedione [3] afforded the corresponding b-diketone 4. Cyclization of this using hydrazine [4] or guanidine [5] resulted in the formation of pyrazole 5 or pyrimidine 6, respectively, bridged with sulfur to the imidazole ring. All these compounds possess a saccharide moiety in the 4 position of the imidazole ring.
The same results were also obtained starting from 3-O-benzyl-1,2-O-isopropylidene-a-D- xylo-pentodialdo-1,4-furanose [6] and methyl 2,3-O-isopropylidene-a-D-ribo-pentodialdo-1,4-furanoside [7] applying the above described reaction sequence. The 1H-, 13C NMR, and mass spectral data of the prepared compounds were also studied.
2 Steiner, B.; Koos, M. submitted for publication.
3 Koos, M. Chem Papers 1994, 48, 108-110.
4 Koos, M.; Steiner, B.; Repas, M. Chem. Papers 1991, 45, 279-286.
5 Brown, D. J.; England, B. T. J. Chem. Soc. (C) 1967, 1922-1927.
6 Kovar, J.; Baer, H. H. Carbohydr. Res. 1975, 39, 19-35.
7 Jones, G. H.; Moffatt, J. G. Methods Carbohydr. Chem. 1972, 6, 315-322.