A simple modification to improve the generality of Terry's 3,4-disubstituted pyrrole synthesis
Instituto de Química, UNAM, Circuito Exterior, Ciudad Universitaria,
Coyoacán 04510, México D.F., México
Abstract
The reaction between 1-benzenesulfonamidopropan-2-one and
electrophilic olefins affords substituted pyrrolidines, that are readily converted into
3,4-disubstituted pyrroles by standard methods.
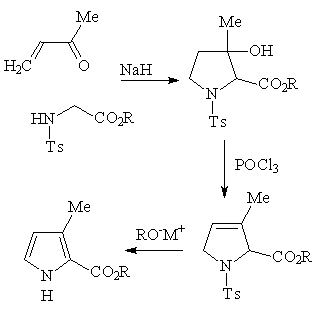
Synthesis of 2,3-disubstituted pyrroles according
to Terry, Jackson, Kenner and Konis
The condensation of an alkyl N-tosyl glycinate with a vinyl ketone in basic
media has been reported (Terry, W.G.; Jackson, A.H., Kenner, G.W.; Konis G., J. Chem. Soc., 1965, 4389) to give substituted pyrrolidinols by tandem Michael
and intramolecular aldol type reactions. Dehydration and base promoted
elimination of the p-toluenesulfinate anion then gives 3-substituted-2-pyrrole
carbolic esters:
Scheme 1
Synthesis of 3,4-disubstituted pyrroles according
to Terry, Jackson, Kenner and Konis
The method can be easily adapted for the preparation of 3,4-disubstituted
pyrroles if one starts from the appropriately substituted enone. However, the
carboxylic ester group in the initially obtained 3,4-disubstituted-2-pyrrole
carboxylic ester should be removed, thus adding two extra steps to the
sequence:
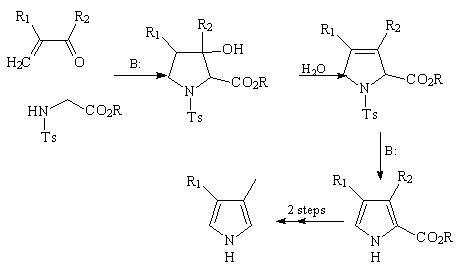
Scheme 2
Incidentally, in this case the R' and R2 substituents can hardly be
synthetically useful functional groups.
Exchange of functional groups, the key to out proposal (I)
Now we wish to report a simple structural modification in the involved
substrates of Scheme 1, which leads directly to 3,4-disubstituted pyrroles
(without the need to remove "extra" groups) and in which at least one
substitutent is functionalized and therefore prone to further synthetic
elaboration.
We reasoned that an exchange in the functional groups in the original
substrates of the Terry method (Scheme 1), would
give a new pair of starting materials (now an a,b-unsaturated ester
and an a-arenesulfonamido ketone) which by the same sequence of reactions
would afford 3,4-disubstituted pyrroles.
Exchange of functional groups, the key to out proposal (II)
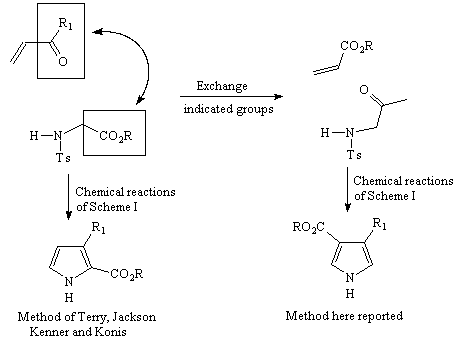
Scheme 3
Hence, this simple modification changes the substitution pattern in the final
product, affording directly the most difficulty accessible 3,4-disubstituted
pyrrole.
Synthesis of 1-benzenesulfonamidopropan-2-one
At present, all our experiments have been performed with the title compound as
the
a-arenesulfonamido ketone component. This compound has been obtained
quite easily as indicated (85% overall yield)
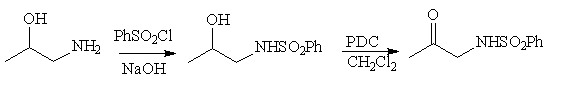
Scheme 4
Spectral data: IR(CHCl3) 3350, 1730,1344,1165,1095 cm-1;
1H NMR (CDCl3, 200 MHz) d 2.10 (s,3H), 3.90 (d,J=4.6,2H;
collapses to a singlet with D2O), 5.40 (broad, NH), 7.55 (m,3H), 7.90 (m,2H);
MS (70eV) m/z (%) 214(M++1,1), 213 (M+, 1), 170 (100),
141 (78), 77 (73), 51 (12), 43 (19).
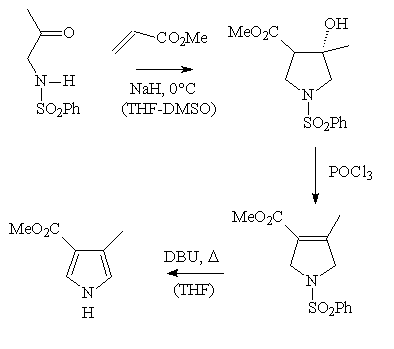
Synthesis of methyl 4-methyl-3-pyrrole carboxyalte
Condensation of 1-benzenesulfonamidopropan-2-one with methyl acrylate in the
presence of NaH (1.1 equiv.) in a 1:1 mixture of THF-DMSO at 0 oC, gave a
crystalline pyrrolidine (mp 110 oC) as a single diastereoisomer. Dehydration
to the d3-pyrroline with POCl3-pyridine and aromatization by
elimination of the benzenesulfinate anion with ButOK or (much simpler) DBU in
refluxing THF, gave the title known (Van Leusen, A.M.; Siderius, H.; Hoogenboom, B.E.; Van Leusen, D. Tetrahedron
Lett., 1972, 5337) pyrrole in ~ 40% overrall yield.
Scheme 5
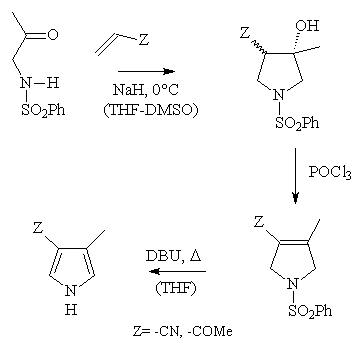
Other 4-methyl-3-substituted pyrroles obtained in this work
Two other electrophilic olefins (acrylonitrile and MVK) have also been used in
the general sequence, affording the corresponding 4-methyl-3-substituted
pyrroles in comparable yields (~ 35-40% overall )
Scheme 6
Conclusion
- With MVK we have found optimal yields in the first step when performed at
low temperature (-78 oC)
- With MVK no 2,3-disubstituted pyrrole has been detected (potentially formed
by enolate equilibration prior to aldol cyclization in the first step)
- In striking contrast to the methyl acrylate case, the corresponding cyclic
aldols with MVK and acrylonitrile are obtained as nearly 1:1 mixtures of
diastereoisomers
- The Terry method and ours, proceed through the
same C3-C4 and N-C5 bond forming processes
- In the Terry method, one starting material
behaves as a double donor reagent (the N-tosyl glycinate) and the other as a
double acceptor reagent (the enone). Both substituents in the resulting pyrrole
come from the enone
- In our modification, each substrate has the double function of being
donor-acceptor reagents and each one contributes with one substituent in the
final pyrrole





