 ECHET96 Article 076: Masatomi Ohno
ECHET96 Article 076: Masatomi Ohno
 reply to question on article 76 by M. Ohno R.Alan Aitken
reply to question on article 76 by M. Ohno R.Alan Aitken
Construction of extended and polymeric 1,3-dithiolane
and tetrathiafulvalene derivatives using cycloaddition of
Bu3P*CS2
School of Chemistry,
University of St. Andrews,
St. Andrews, Fife, UK KY16 9ST
Addition to strained double bonds and the Wittig reaction
The reaction of the zwitterionic tributylphosphoniodithioformate
1 with a number of alkenes has been investigated. No cycloaddition was
observed with cyclohexene, styrene or stilbene. With norbornene a bright pink
solid 2 was rapidly formed. The structure of this and its composition
as a 1:1 adduct + CS2 were clear from analytical
and spectroscopic data.
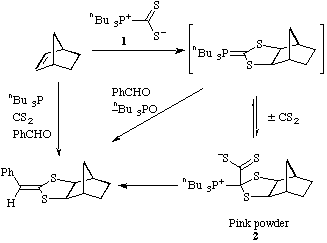
In particular the 13C NMR
spectrum clearly showed the unusual structure 2. The intermediate ylide
can be successfully intercepted by carrying out the reaction in the presence of
benzaldehyde producing the new tricyclic dithiolane. The pink adduct 2
also reacts with benzaldehyde to give the same product. Most conveniently the
one pot reaction of the four simple starting materials also gave this complex
product directly in moderate yields. Norbornadiene gave similar results:
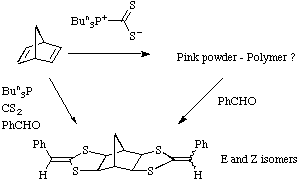
We have previously reported that this reaction can be carried out on a range of
bicyclo[2.2.1]alkenes readily available from the Diels-Alder reaction of
cyclopentadiene.1 In the remainder of this presentation, we will
concentrate on more recent extensions of this chemistry.
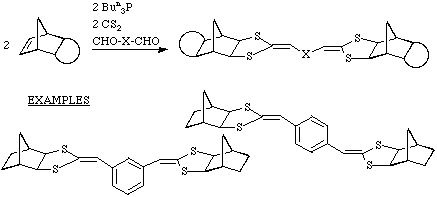
Extension to dialdehydes
The reaction shown above for benzaldehyde also works with acetaldehyde
and isobutyraldehyde but apparently not with simple ketones such as acetone.
The scope of the reaction was broadened by the use of dialdehydes leading to
the products shown.
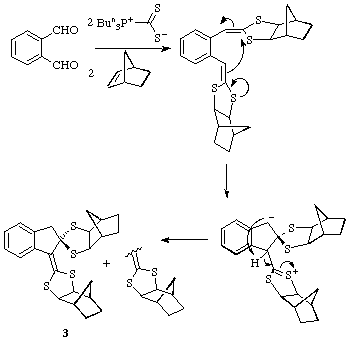
For the ortho-substituted phthalic dialdehyde, interaction of the two
alkylidenedithiolane functions occurs as shown to afford the unusual polycyclic
product 3 as a 1:1 mixture of isomers (enantiomers). Its structure has
been established by an X-ray structure determination.
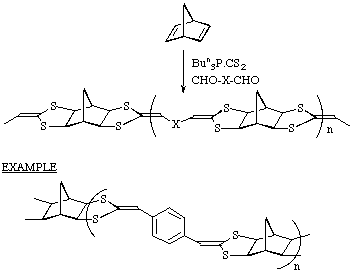
Extension to polymeric systems
By combining a dialdehyde with norbornadiene a novel class of polymers can be
obtained.
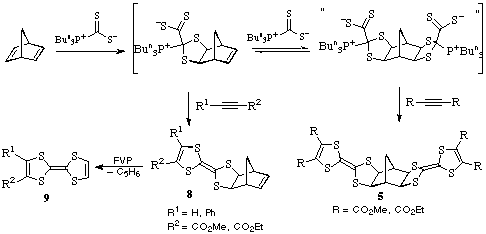
Reaction of 2 with acetylenic esters - an important new area
The pink adduct 2 also reacts with acetylenic esters such as dimethyl
acetylenedicarboxylate by cycloaddition with elimination of tributylphosphine
to give the tetracyclic dihydrotetrathiafulvalene structures 4.
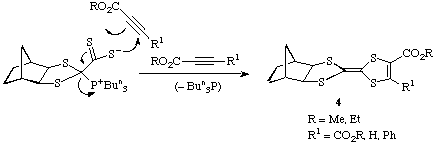
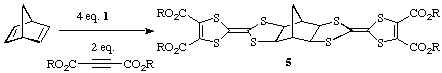
The pink adduct derived from norbornadiene reacts similarly to give the
corresponding hexacyclic products 5.
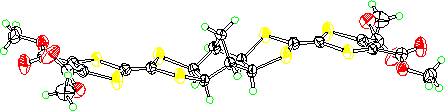
The X-ray structure of 5 (R = Me) shows that although the two dithiolane
rings are exo there is a bend at the four central sulfurs to make the
molecule approximately planar overall. Because of this they pack randomly
with respect to whether the norbornane bridge is up or down.
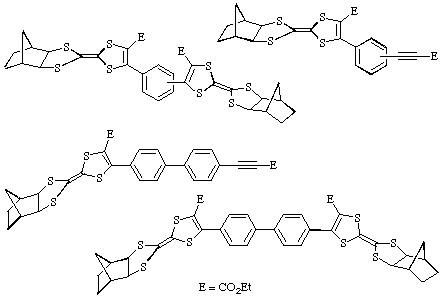
The reaction of 2 with bis(acetylenic) esters leads to the
formation of a variety of adducts such as those shown:
Potential applications
Possible applications of the above structures include the formation of
charge-transfer complexes with suitable organic acceptors such as
tetracyano-p-quinodimethane (TCNQ) or with suitable inorganic anions
(e.g. I3-, PF6-,
ClO4-) for use as potential organic conductors or
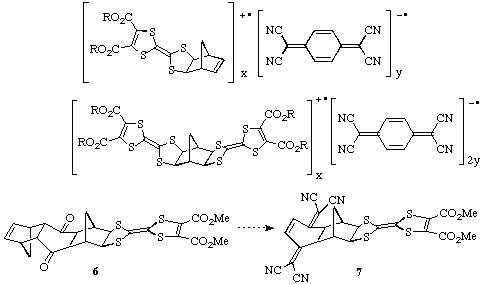
superconductors. Some examples of the types of complexes potentially accessible
are shown below. In addition, by starting from the bis-cyclopentadiene adduct
of benzoquinone, we have reached the stage of 6 on the way to the
compound 7 which has the donor and acceptor groups in the same molecule.
It will be noticed that all the systems mentioned up to now have been of the
dihydro-TTF type which lack full conjugation. An important recent development
has allowed us to use this method to get unsymmetrically substituted
tetrathiafulvalenes.
Formation of substituted tetrathiafulvalenes
As mentioned in the above reaction, of the norbornadiene /
Bu3P*CS2 adduct with DMAD or DEAD gives the extended
bis-dihydro-TTF structures 5. However when the same compound reacts
with methyl or ethyl propiolate or phenylpropiolate, i.e. less
electron-deficient alkynes, the dihydro-TTF is formed on one side of the
norbornene but the other remains as the double bond. These products 8
are important since they readily undergo very ready retro-Diels-Alder reaction
upon FVP to give cyclopentadiene and the TTFs 9 shown.
In fact the "mono" products are also formed in isolated yields of ~10% for
DMAD and DEAD together with the main "bis" products in 25-30 % yield. This
implies that various species are present and available for reaction from
interaction of 1 with norbornadiene. Most recently the "mono" Wittig
product has also been isolated as a minor byproduct in the reaction with
isobutyraldehyde.
The pyrolysis in all cases proceeds in almost quantitative yield. The ability
to prepare the specifically mono- and di-substituted TTFs 9 in just two
simple steps from the alkyne, norbornadiene, Bu3P and
CS2 may be one of the most useful discoveries from this research programme.
Acknowledgements
We thank the EPSRC for a Research Grant (GR/J 38895) under the Innovative
Polymer Synthesis Initiative and Dr Philip Lightfoot for the X-ray Structure
of 5.
- R. A. Aitken, T. Massil and S. V. Raut, J. Chem. Soc., Chem. Commun.,
1994, 2603.