December 2nd, 2017
For around 16 years, Floyd Romesberg’s group has been exploring un-natural alternatives (UBPs) to the Watson-Crick base pairs (C-G and A-T) that form part of the genetic code in DNA. Recently they have had remarkable success with one such base pair, called X and Y (for the press) and dNaMTP and d5SICSTP (in scholarly articles).[1],[2] This extends the genetic coding from the standard 20 amino acids to the possibility of up to 172 amino acids. Already, organisms engineered to contain X-Y pairs in their DNA have been shown to express entirely new (and un-natural) proteins.
Read the rest of this entry »
References
- A.W. Feldman, M.P. Ledbetter, Y. Zhang, and F.E. Romesberg, "Reply to Hettinger: Hydrophobic unnatural base pairs and the expansion of the genetic alphabet", Proceedings of the National Academy of Sciences, vol. 114, 2017. https://doi.org/10.1073/pnas.1708259114
- D.A. Malyshev, K. Dhami, H.T. Quach, T. Lavergne, P. Ordoukhanian, A. Torkamani, and F.E. Romesberg, "Efficient and sequence-independent replication of DNA containing a third base pair establishes a functional six-letter genetic alphabet", Proceedings of the National Academy of Sciences, vol. 109, pp. 12005-12010, 2012. https://doi.org/10.1073/pnas.1205176109
Tags: Base pair, Biology, DNA, Floyd Romesberg, Gene, genetic code, Genetics, Molecular biology, Molecular genetics, Nucleic acids, Nucleotide, Synthetic genomics
Posted in Interesting chemistry | 3 Comments »
November 28th, 2017
I started this story by looking at octet expansion and hypervalence in non-polar hypercoordinate species such as S(-CH3)6, then moved on to S(=CH2)3. Finally now its the turn of S(≡CH)2.‡
Read the rest of this entry »
Tags: 1-Decyne, CH2, chemical bonding, free energy, G. N. Lewis, Lewis structure, Music, Octet
Posted in Historical, Hypervalency | 3 Comments »
November 27th, 2017
Previously: “Non-polar” species such as SeMe6, SMe6, ClMe3, ClMe5 all revealed interesting properties for the Se-C, S-C or Cl-C “single” bonds. The latter two examples in particular hinted at internal structures for these single bonds, as manifested by two ELF basins for some of the bonds. Here I take a look at the related molecule where a formal double bond between carbon and the central sulfur atom replacing the single-bond might also hint at octet expansions and hypervalence.
Read the rest of this entry »
Tags: Chemical bond, chemical bonding, Chemical polarity, Chemistry, double bond, Hypervalent molecule, Nature, single bond, Tetravalence, Valence
Posted in Hypervalency | No Comments »
November 20th, 2017
Following on from discussing octet expansion in species such as SeMe6, ClMe3 and ClMe5, I felt impelled to return to SF6, often used as an icon for hypervalence.
Read the rest of this entry »
Tags: chemical bonding, Chemistry, Hypervalent molecules, Octet, Sulfur hexafluoride
Posted in Hypervalency | 7 Comments »
November 14th, 2017
PIDapalooza is a new forum concerned with discussing all things persistent, hence PID. You might wonder what possible interest a chemist might have in such an apparently arcane subject, but think of it in terms of how to find the proverbial needle in a haystack in a time when needles might look all very similar. Even needles need descriptions, they are not all alike and PIDs are a way of providing high quality information (metadata) about a digital object.
Read the rest of this entry »
Tags: chemist, computing, Information, Information science, Knowledge representation, librarian, Needle, PID
Posted in Chemical IT | No Comments »
November 12th, 2017
A few years back, I took a look at the valence-shell electron pair repulsion approach to the geometry of chlorine trifluoride, ClF3 using so-called ELF basins to locate centroids for both the covalent F-Cl bond electrons and the chlorine lone-pair electrons. Whereas the original VSEPR theory talks about five “electron pairs” totalling an octet-busting ten electrons surrounding chlorine, the electron density-based ELF approach located only ~6.8e surrounding the central chlorine and no “octet-busting”. The remaining electrons occupied fluorine lone pairs rather than the shared Cl-F regions. Here I take a look at ClMe3, as induced by the analysis of SeMe6.
Read the rest of this entry »
Tags: Chemical bond, chemical bonding, Chemistry, Chlorine, Covalent bond, Lone pair, Oxidizing agents, Quantum chemistry, Stereochemistry, Valence, VSEPR theory
Posted in Chemical IT, Hypervalency | 5 Comments »
November 7th, 2017
One thread that runs through this blog is that of hypervalency. It was therefore nice to come across a recent review of the concept[1] which revisits the topic, and where a helpful summary is given of the evolving meanings over time of the term hypervalent. The key phrase “it soon became clear that the two principles of the 2-centre-2-electron bond and the octet rule were sometimes in conflict” succinctly summarises the issue. Two molecules that are discussed in this review caught my eye, CLi6 and SeMe6. The former is stated as “anomalous in terms of the Lewis model“, but as I have shown in an earlier post, the carbon is in fact not anomalous in a Lewis sense because of a large degree of Li-Li bonding. When this is taken into account, the Lewis model of the carbon becomes more “normal”. Here I take a look at the other cited molecule, SeMe6.
Read the rest of this entry »
References
- M.C. Durrant, "A quantitative definition of hypervalency", Chemical Science, vol. 6, pp. 6614-6623, 2015. https://doi.org/10.1039/c5sc02076j
Tags: chemical bonding, City: Aachen, Hypervalent molecule, Molecular geometry
Posted in Hypervalency | 4 Comments »
October 24th, 2017
An N-B single bond is iso-electronic to a C-C single bond, as per below. So here is a simple question: what form does the distribution of the lengths of these two bonds take, as obtained from crystal structures?
Read the rest of this entry »
Tags: bond, Bond valence method, Chemical bond, chemical bonding, Chemistry, Covalent bond, crystal structure, Nature, Quantum chemistry, search query
Posted in crystal_structure_mining | 2 Comments »
October 5th, 2017
We have heard a lot about OA or Open Access (of journal articles) in the last five years, often in association with the APC (Article Processing Charge) model of funding such OA availability. Rather less discussed is how the model of the peer review of these articles might also evolve into an Open environment. Here I muse about two experiences I had recently.
Read the rest of this entry »
Tags: Academic publishing, article processing charge, author, Company: Facebook, Company: Publons, Company: Twitter, editor, Electronic publishing, Entertainment/Culture, Hybrid open access journal, Internet giants, OA, Open access, Organic Syntheses, Public sphere, Publishing, Scholarly communication, search engines, Social Media & Networking, Technology/Internet
Posted in Chemical IT, General | 5 Comments »
October 1st, 2017
I noted in my WATOC conference report a presentation describing the use of calculated reaction barriers (and derived rate constants) as mechanistic reality checks. Computations, it was claimed, have now reached a level of accuracy whereby a barrier calculated as being 6 kcal/mol too high can start ringing mechanistic alarm bells. So when I came across this article[1] in which calculated barriers for a dyotropic ring expansion observed under mild conditions in dichloromethane as solvent were used to make mechanistic inferences, I decided to explore the mechanism a bit further.
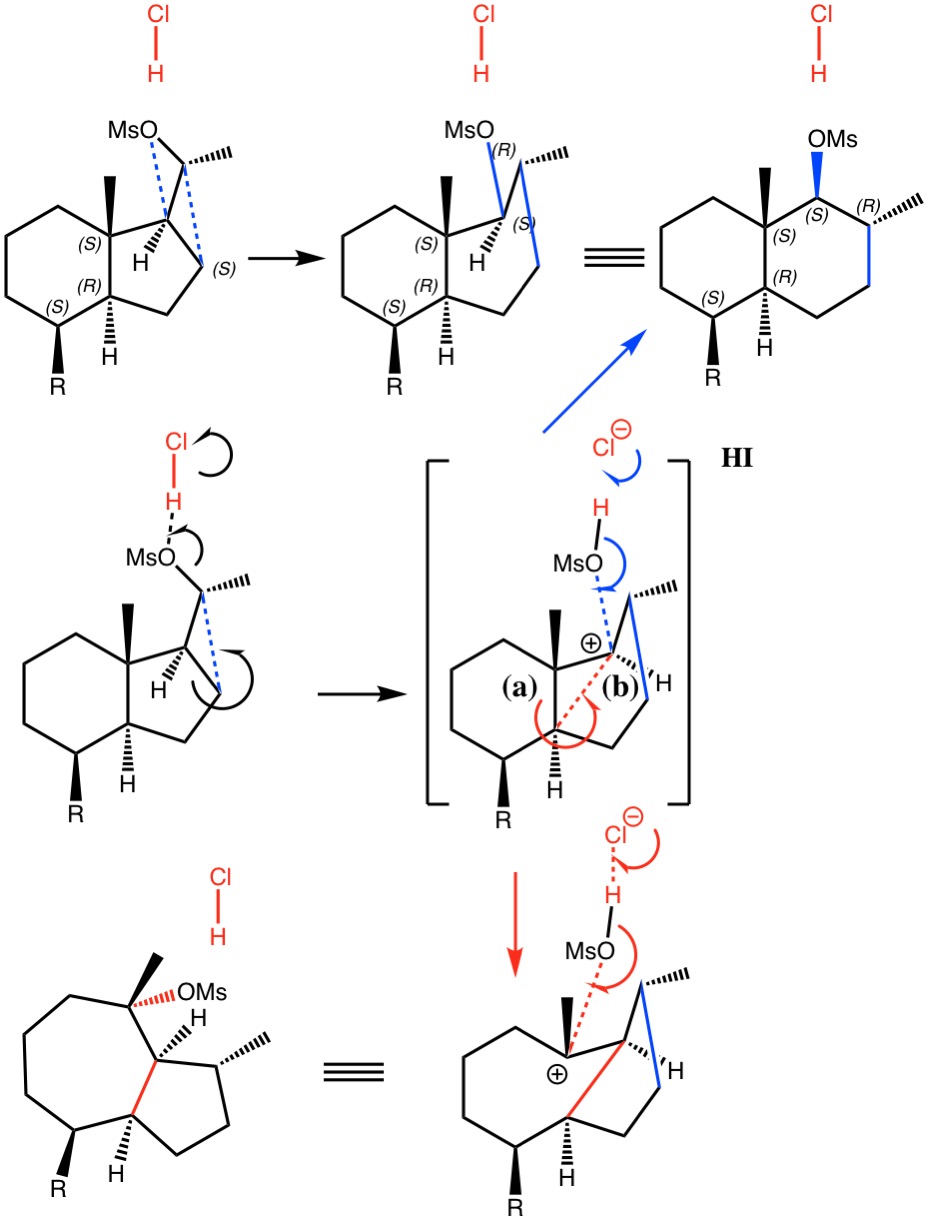
Read the rest of this entry »
References
- H. Santalla, O.N. Faza, G. Gómez, Y. Fall, and C. Silva López, "From Hydrindane to Decalin: A Mild Transformation through a Dyotropic Ring Expansion", Organic Letters, vol. 19, pp. 3648-3651, 2017. https://doi.org/10.1021/acs.orglett.7b01621
Tags: animation, bicyclic ring product, energy derivative gradient norm, energy profile, final non-ionic product, Organic chemistry, possible products, potential energy surface, realistic model for the reaction
Posted in pericyclic, reaction mechanism | 3 Comments »
