My previous post introduced the interesting guts of taxol. Two different isomers can exist, and these are called atropisomers; one has the carbonyl group pointing up, the other down. The barrier to their interconversion in this case is generated by a rotation about the two single bonds connecting the carbonyl group to the rest of the molecule. Introductory chemistry tells us that the barrier for rotation about such single bonds is low (i.e. fast at room temperature). But is that true here?
Atropisomerism in Taxol. An apparently simple bond rotation?
November 1st, 2011Blogbooks, e-books and future proofing chemical diagrams.
October 31st, 2011Most of the chemical structure diagrams in this blog originate from Chemdraw, which seems to have been around since the dawn of personal computers! I have tended to use this program to produce JPG bitmaps for the blog, writing them out in 4x magnification, so that they can be scaled down for display whilst retaining some measure of higher resolution if needed for other purposes. These other purposes might be for e.g. the production of e-books (using Calibre), the interesting Blog(e)book format offered as a service by Feedfabrik, or display on mobile tablets where the touch-zoom metaphor to magnify works particularly well. But bitmap images are not really well future proofed for such new uses. Here I explore one solution to this issue.
Computers 1967-2011: a personal perspective. Part 4. Moore’s Law and Molecules.
October 28th, 2011Moore’s law describes a long-term trend in the evolution of computing hardware, and it is often interpreted in terms of processing speed. Here I chart this rise in terms of the size of computable molecules. By computable I mean specifically how long it takes to predict the geometry of a given molecule using a quantum mechanical procedure.
The colour of purple is … not orange but mauve?
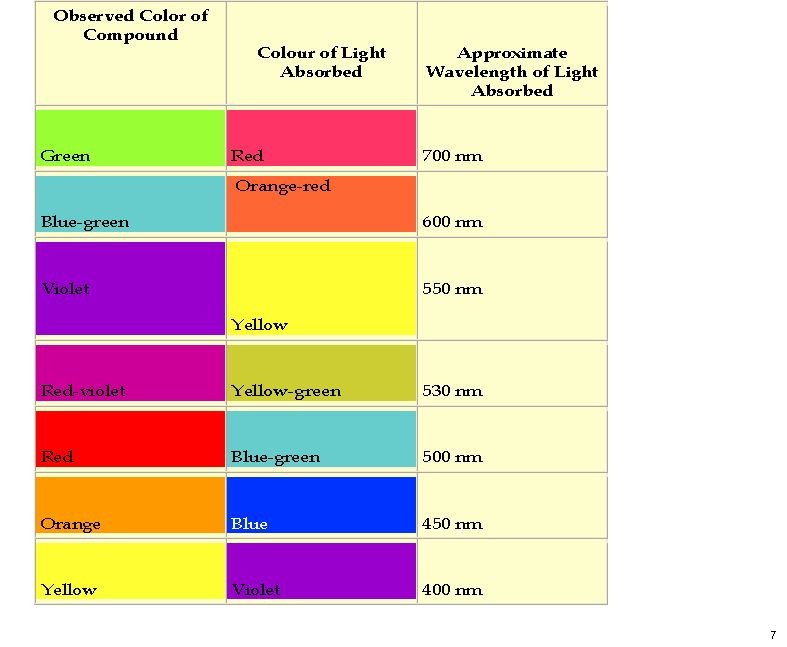
October 26th, 2011My previous post on the topic of mauveine left the outcome dangling. Put simply, λmax is measured at about 549nm for mauveine A, but was calculated at about 440nm using a modern method for predicting colour (TD-DFT). According to the colour table below, that would make it orange, not mauve. Can the theoretical prediction be out by 110nm, or might it be the structure of the molecule itself that has been wrongly described?
The SN1 Mechanism for a third time. Exploration of the intrinsic reaction coordinate.
October 25th, 2011As the title hints, I have been here before. The SN1 solvolysis mechanism of t-butyl chloride was central to the flourishing of physical organic chemistry from the 1920s onwards, and it appears early on in most introductory lecture courses and text books. There we teach that it is a two-stage mechanism. Firstly the C-Cl bond heterolyses to form a stable tertiary carbocation intermediate, which in a second stage reacts with nucleophile (water) to form e.g. t-butanol. This is contrasted with the SN2 mechanism, where these two stages are conflated into a single concerted process, involving no intermediates. Here I explore an intrinsic reaction coordinate for the hydrolysis of t-butyl chloride which attempts to tease out whether this simple picture is realistic.
Science publishers (and authors) please take note.
October 24th, 2011I have for perhaps the last 25 years been urging publishers to recognise how science publishing could and should change. My latest thoughts are published in an article entitled “The past, present and future of Scientific discourse” (DOI: 10.1186/1758-2946-3-46). Here I take two articles, one published 58 years ago and one published last year, and attempt to reinvent some aspects. You can see the result for yourself (since this journal is laudably open access, and you will not need a subscription). The article is part of a special issue, arising from a one day symposium held in January 2011 entitled “Visions of a Semantic Molecular Future” in celebration of Peter Murray-Rust’s contributions over that period (go read all 15 articles on that theme in fact!).
Historical detective stories: colourful crystals.
October 21st, 2011Organic chemists have been making (more or less pure) molecules for the best part of 180 years. Occasionally, these ancient samples are unearthed in cupboards, and then the hunt for their origin starts. I have previously described tracking down the structure of a 120 year-old sample of a naphthalene derivative. But I visited a colleague's office today, and recollected having seen a well-made wooden display cabinet there on a previous visit. Today I took a photo of one of the samples:
The perception of stereochemistry. A challenging case.
October 18th, 2011Most representational chemistry generated on a computer requires the viewer to achieve a remarkably subtle transformation in their mind from two to three dimensions (we are not quite yet in the era of the 3D iPad!). The Cahn-Ingold-Prelog convention was a masterwork (which won the Nobel prize). It is shown in action for the molecule on the left below. The CIP notation was actually generated by Chemdraw, and required a fair sprinkling of wedged and hashed bonds to (try to) remove stereoambiguity and generate the labels (try it for yourself). As part of a lecture course on pericyclic reactions, I tell the students that the reaction involves a [1,3] sigmatropic migration of the red carbon and that this migration proceeds with inversion of configuration at this migrating carbon (as the selection rules require). Perceiving what the correct CIP product label should be (with inferred stereochemical labels, resolving ? into either R or S) is IMHO one of the most difficult conceptual experiences in all of organic chemistry. I have over the years struggled to find a way of revealing this in lecture notes (these struggles with the “lecture notes” will be the topic of a future post here). However, I think I may have finally cracked it; my solution is set out below!
Mechanism of the reduction of a carboxylic acid by borane: revisited and revised.
October 16th, 2011I asked a while back whether blogs could be considered a serious form of scholarly scientific communication (and so has Peter Murray-Rust more recently). A case for doing so might be my post of about a year ago, addressing why borane reduces a carboxylic acid, but not its ester, where I suggested a possible mechanism. Well, colleagues have raised some interesting questions, both on the blog itself and more silently by email to me. As a result, I have tried to address some of these questions, and accordingly my original scheme needs some revision! This sort of iterative process of getting to the truth with the help of the community (a kind of crowd-sourced chemistry) is where I feel blogs do have a genuine role to play.
Bonds.
October 13th, 2011Bonds are a good example of something all chemists think they can recognise when they see them. But they are also remarkably dependent on context. We are running a molecular modelling course at the moment, and I found myself explaining to someone how very context-sensitive they can be. I thought it might be useful to collect my thoughts here. Read the rest of this entry »