I love experiments where the insight-to-time-taken ratio is high. This one pertains to exploring the coordination chemistry of the transition metal region of the periodic table; specifically the tetra-coordination of the series headed by Mn-Ni. Is the geometry tetrahedral, square planar, or other? One can get a statistical answer in about ten minutes.
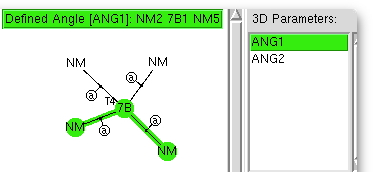 The (CCDC database) search definition required is shown above. The central atom defines the column of the period table, it is specified to have precisely four other atoms bonded to it, which can be any other element. These four bonds are specified as acyclic (to avoid any bias introduced by rings). And two angles are defined subtending the central atom. And off we go, defining on the way that the hits must be refined to an R-factor of < 0.05, have no disorder, and no errors.
The (CCDC database) search definition required is shown above. The central atom defines the column of the period table, it is specified to have precisely four other atoms bonded to it, which can be any other element. These four bonds are specified as acyclic (to avoid any bias introduced by rings). And two angles are defined subtending the central atom. And off we go, defining on the way that the hits must be refined to an R-factor of < 0.05, have no disorder, and no errors.
Archive for April, 2014
Ribulose-1,5-bisphosphate + carbon dioxide → carbon fixation!
Sunday, April 20th, 2014Ribulose-1,5-bisphosphate reacts with carbon dioxide to produce 3-keto-2-carboxyarabinitol 1,5-bisphosphate as the first step in the biochemical process of carbon fixation. It needs an enzyme to do this (Ribulose-1,5-bisphosphate carboxylase/oxygenase, or RuBisCO) and lots of ATP (adenosine triphosphate, produced by photosynthesis). Here I ask what the nature of the uncatalysed transition state is, and hence the task that might be facing the catalyst in reducing the activation barrier to that of a facile thermal reaction. I present my process in the order it was done‡.
More (blog) connections spotted. Something new about diphenyl magnesium?
Thursday, April 17th, 2014I have just noticed unexpected links between two old posts, one about benzene, one about diphenyl magnesium and a link to August Kekulé.†
Enantioselective epoxidation of alkenes using the Shi Fructose-based catalyst. An undergraduate experiment.
Tuesday, April 15th, 2014The journal of chemical education can be a fertile source of ideas for undergraduate student experiments. Take this procedure for asymmetric epoxidation of an alkene.[1] When I first spotted it, I thought not only would it be interesting to do in the lab, but could be extended by incorporating some modern computational aspects as well.
References
- A. Burke, P. Dillon, K. Martin, and T.W. Hanks, "Catalytic Asymmetric Epoxidation Using a Fructose-Derived Catalyst", Journal of Chemical Education, vol. 77, pp. 271, 2000. https://doi.org/10.1021/ed077p271
Artemisinin: are stereo-electronics at the core of its (re)activity?
Sunday, April 13th, 2014Around 100 tons of the potent antimalarial artemisinin is produced annually; a remarkable quantity given its very unusual and fragile looking molecular structure (below). When I looked at this, I was immediately struck by a thought: surely this is a classic molecule for analyzing stereoelectronic effects (anomeric and gauche). Here this aspect is explored.
A connected world (journals and blogs): The benzene dication.
Thursday, April 10th, 2014Science is rarely about a totally new observation or rationalisation, it is much more about making connections between known facts, and perhaps using these connections to extrapolate to new areas (building on the shoulders of giants, etc). So here I chart one example of such connectivity over a period of six years.
What is the best way of folding a straight chain alkane?
Sunday, April 6th, 2014In the previous post, I showed how modelling of unbranched alkenes depended on dispersion forces. When these are included, a bent (single-hairpin) form of C58H118 becomes lower in free energy than the fully extended linear form. Here I try to optimise these dispersion forces by adding further folds to see what happens.