After around 40 posts here, I decided to take a look at the whole effort and ask some questions. For example
(more…)
Archive for the ‘Chemical IT’ Category
How long will a blog last? ArchivePress
Saturday, January 9th, 2010Spotting the unexpected: Anomeric effects
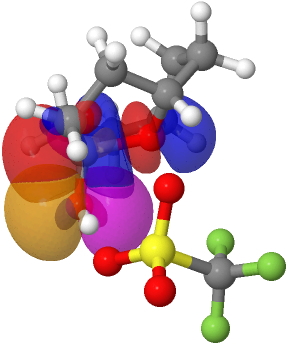
Friday, September 18th, 2009Chemistry can be very focussed nowadays. This especially applies to target-driven synthesis, where the objective is to make a specified molecule, in perhaps as an original manner as possible. A welcome, but not always essential aspect of such syntheses is the discovery of new chemistry. In this blog, I will suggest that the focus on the target can mean that interesting chemistry can get over-looked (or if observed, not fully exploited in subsequent publications). Taking a synthesis-oriented publication at (almost) random entitled Synthesis of 1-Oxadecalins from Anisole Promoted by Tungsten (DOI: 10.1021/ja803605m) which appeared in 2008, the following molecule appears as one of the (many) intermediates.
The Fragile Web
Monday, August 31st, 2009One of the many clever things that clever people can do with the Web is harvest it, aggregate it, classify it etc. Its not just Google that does this sort of thing! Egon Willighagen is one of those clever people. He runs the Chemical blogspace which does all sorts of amazing things with blogs.
A lab in a backpack
Friday, April 3rd, 2009We recently developed a new computational chemistry practical laboratory here at Imperial College. I gave a talk about it at the recent ACS meeting in Salt Lake City. If you want to see the details of the lab, do go here. The talk itself contains further links and examples. Perhaps here I can quote only the final remark, namely that computational chemistry can now provide chemical accuracy for many problems, including spectroscopy and mechanism, and that the basic tools for doing it can easily be carried around in a backpack! Or, perhaps in the not to distant future, an iPhone!
On the importance of Digital repositories in Chemistry
Friday, April 3rd, 2009The preceeding blog entries contain stories about chemical behaviour. If you have clicked on the diagrams, you may even have gotten a Jmol view of the relevant molecules popping up. But if you are truly curious, you may even have the urge to acquire the relevant 3D information about the molecule, and play with it yourself. Even after 15 years of the (chemical) Web, this can be distressingly difficult to achieve (or can it be that it is only myself who wishes to view molecules in their native mode?). Thus the standard mechanism is to seek out on journal pages that disarming little entry entitled supporting information and to hope that you might find something useful embedded there. Embedded is the correct description, since the information is often found within the confines of an Acrobat file, and has to be extracted from there. Indeed, that is what I had to resort to in order to write one of the blog entries below. I ground my teeth whilst doing so.
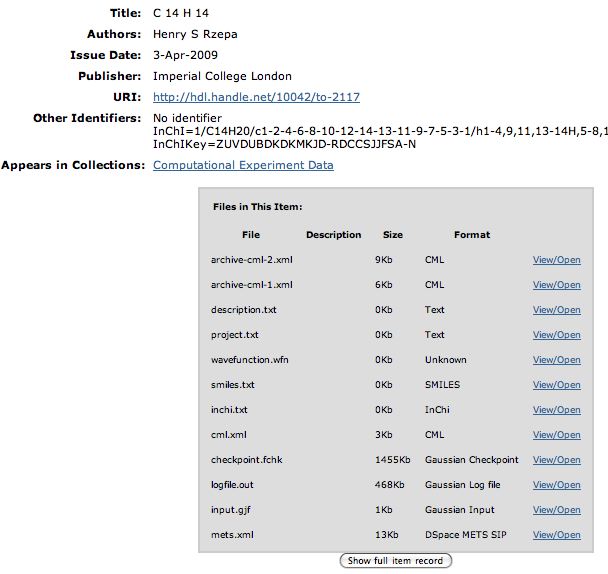
So is there a better way? We think so! The digital repository. If you click on this you should see the entry directly. What can you do there? Well, if you have suitable programs, you can download eg a Checkpoint file of the calculation that created the molecule model and re-activate it there. Or you can download just the CML file for viewing in any CML-compliant program (such as e.g. Jmol). Or you can check up on the InCHi string or the InChI Key of the molecule.